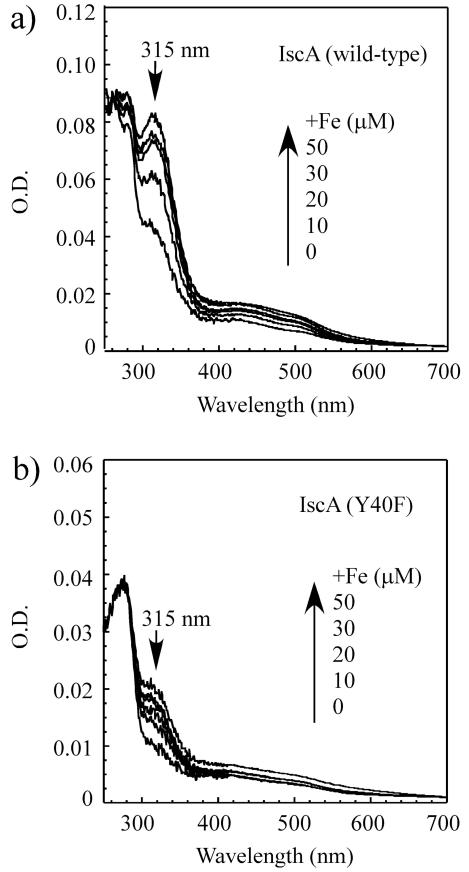
Fig. 4.
IscA mutant Y40F has a diminished iron binding activity. a), purified wild-type IscA dimer (25 μM) was incubated with indicated concentrations of Fe(NH4)2(SO4)2 in the presence of dithiothreitol (2 mM) at room temperature for 20 min, followed by re-purification of protein. Spectra were calibrated to the same amplitude of the absorption peak at 260 nm of the IscA sample after reconstitution with two-fold excess of iron. b), same as in a), except IscA mutant Y40F dimer (25 μM) was used. Spectra were calibrated to the same amplitude of the absorption peak at 260 nm of the Y40F sample after reconstitution with two-fold excess of iron.