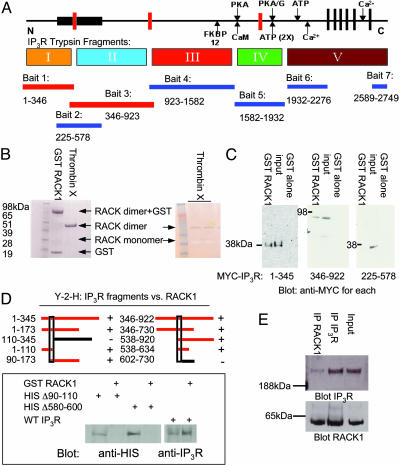
Fig. 1.
RACK1 binds to two distinct sites in the N terminus of IP3R. (A) Schematic depiction of the functional domains of the IP3R, including the ligand-binding (bait 2), modulatory and transmembrane regions, and trypsin digest (I–V) domains. The seven regions used as bait in the yeast two-hybrid screen also are indicated below the protein and the corresponding amino acids (rat type 1 sequence). When RACK1 was screened against all IP3R baits, only baits 1 and 3 (N terminus of IP3R containing the ligand-binding core) supported growth on selective media. (B Left) In vitro Coomassie stain of GST-fusion protein binding of purified RACK1 to glutathione Sepharose and free RACK1 that has been cleaved from GST by using biotinylated thrombin followed by clearing with streptavidin beads. (Right) Silver stain of free RACK1 running as a monomer (negative stain) and a dimer (positive stain). (C) GST pull-down assays with HEK293 cells expressing MYC-tagged IP3R fragments, passed over GST-RACK1 columns. Western blot analysis with anti-MYC antibody demonstrates IP3R fragments that can bind to purified RACK1. (D Upper) Yeast two-hybrid analysis (Y-2-H) for RACK1-binding sites. Red bars depict yeast two-hybrid analysis baits capable of interacting with RACK1. (Lower) Full-length HIS-tagged WT and mutant IP3R were expressed in COS7 cells and passed over GST-RACK1 columns. Western blot analysis with anti-HIS antibody demonstrates IP3R mutants are incapable of binding to RACK1. (E) Coimmunoprecipitation experiments from PC12 cell lysates of endogenous RACK1 and type 1 IP3R, visualized by Western blot analysis.