Figure 4.
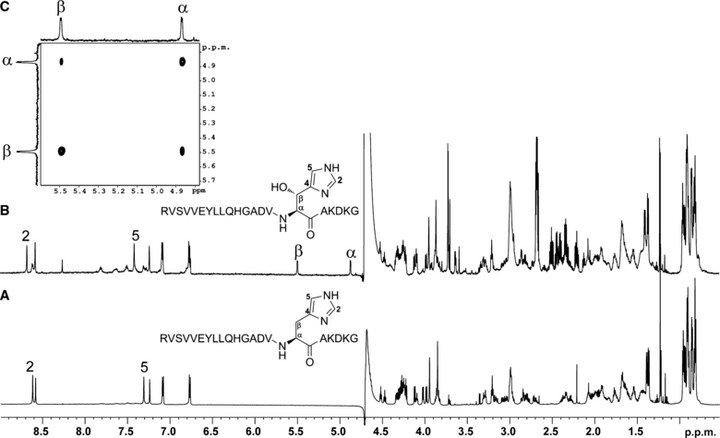
FIH catalysed His-hydroxylation occurs at the β-position. Hydroxylated TNKS2 538–558 peptide (RVSVVEYLLQHGADVHAKDKG) was produced by incubation with FIH under standard assay conditions (hydroxylated to ∼ 75% as assessed by MALDI-TOF analyses), LC-MS purified and analysed by NMR spectroscopy. (B) 1H NMR spectrum of the hydroxylated TNKS2 538–558 peptide in 2H2O. The resonances at 4.87 and 5.50 ppm, which are absent in the 1H NMR spectrum of the nonhydroxylated TNKS2 538–558 peptide (A), are ascribed to the α- and β-proton, respectively, of the hydroxylated His residues. The resonances for the imidazole ring protons (at positions 2 and 5) are deshielded in the spectrum of the hydroxylated peptide compared to that of the nonhydroxylated. (C) 2D 1H–1H COSY spectrum of the hydroxylated TNKS2 538–558 peptide in 2H2O indicating the 1H–1H correlation between resonances arising from the α- and β-hydrogens of the hydroxylated His residue.
