Figure 7.
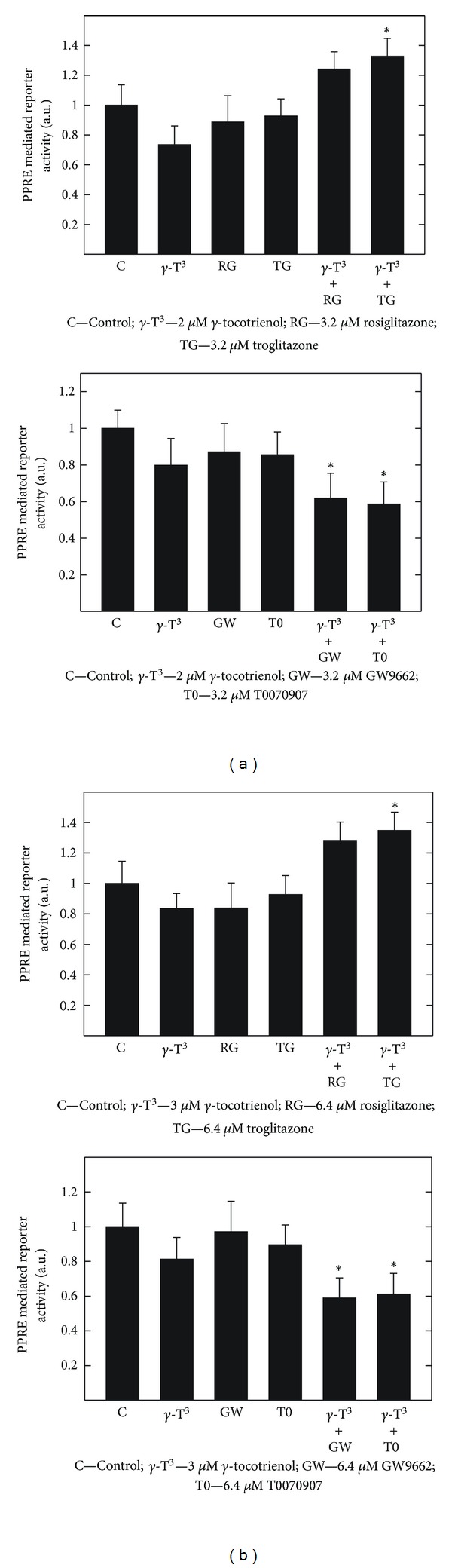
Luciferase assay was performed on (a) MCF-7 and (b) MDA-MB-231 human breast cancer cells. The cells were initially plated at a density of 2 × 104 cells/well in 96-well plates. Cells were then transfected by adding 32 ng of PPRE X3-TK-luc and 3.2 ng of renilla luciferase plasmid in 0.8 μL of lipofectamine 2000 transfection reagent. Following a 6-h incubation period, MCF-7 cells were treated with control or treatment media containing 0–2 μM γ-tocotrienol, 0–3.2 μM rosiglitazone, 0–3.2 μM troglitazone, 0–3.2 μM GW9662, or 0–6.4 μM T0070907 alone or in combination. MDA-MB-231 cells were initially plated in a similar manner and treated with control or treatment media containing 0–3 μM γ-tocotrienol, 0–6.4 μM rosiglitazone, 0–6.4 μM troglitazone, 0–6.4 μM GW9662, or 0–6.4 μM T0070907 alone or in combination. All cells were fed fresh treatment media every other day for 4-day incubation period. Afterwards, cells were lysed with 75 μL of passive lysis buffer and treated according to manufacturer's instructions using the dual-glo luciferase assay system. Results were calculated as raw luciferase units divided by raw renilla units. Vertical bars indicate PPRE mediated reporter activity ± SEM (arbitrary units) in each treatment group. *P < 0.05 as compared with vehicle-treated controls.
