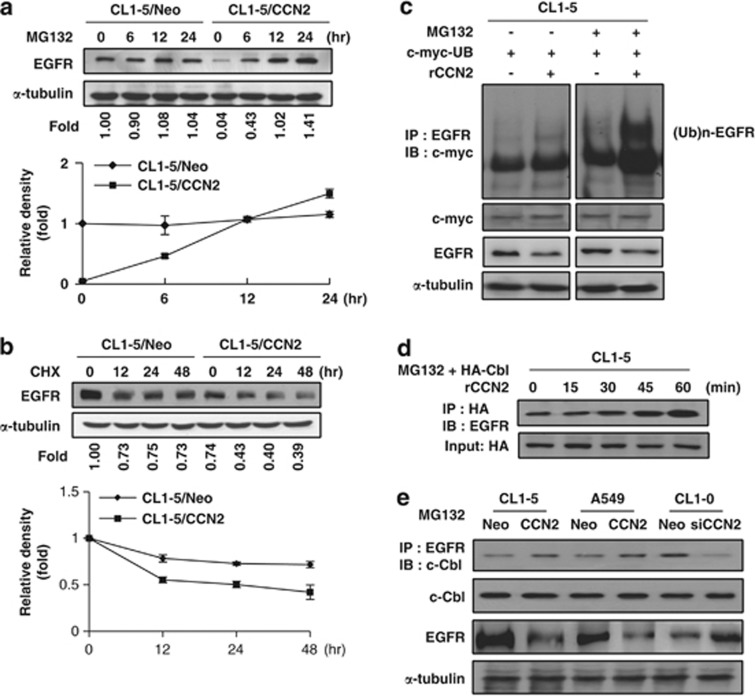
Figure 3.
CCN2 accelerates ubiquitination-dependent EGFR degradation in lung adenocarcinoma cells. (a) CL1-5/CCN2 and CL1-5/Neo stably transfected clones were treated with 10 μM MG132. Cell lysates are subjected to western blotting with anti-EGFR and monoclonal anti-human α-tubulin as an internal control. Relative ratios of EGFR protein signal intensity to that of the internal control are shown below the graph (top). EGFR levels from CL1-5/Neo are assigned as onefold at 0 min time. Means and S.E.M. of four experiments performed in duplicate are shown (bottom). (b) CCN2 transfectants are treated with either vehicle (0.01% dimethyl sulfoxide) or 10 μM cycloheximide (CHX) for the indicated time. Proteins (CL1-5/Neo=50 μg per lane; CL1-5/CCN2=100 μg per lane) were subjected to western blotting using anti-EGFR and anti-α-tubulin (top). Densitometry quantification of EGFR by western blot following normalization to α-tubulin levels. EGFR levels from CL1-5/Neo or CL1-5/CCN2 cells were arbitrarily assigned a value of 100% at 0 min time. Means and S.E.M. of three experiments performed in duplicate were shown (bottom). (c) With or without MG132 or rCCN2 protein treatment, cell lysates of CL1-5 cells transiently transfected with c-myc-ubiquitin-expressing plasmids were immunoprecipitated with anti-EGFR and immunoblotted with monoclonal mouse anti-c-myc-ubiquitin. Internal controls were performed by α-tubulin and c-myc total protein. (d) WB-IP analysis of CL1-5 transiently transfected HA-tag c-Cbl-expressing plasmids with MG132. Cell lysates were immunoprecipitated with anti-HA and immunoblotted with anti-EGFR. HA was used as loading control. (e) WB-IP analysis of CCN2-stable transfectants with MG132 treatment. Cell lysates were immunoprecipitated with anti-EGFR and immunoblotted with anti-c-Cbl. Input controls were including total c-Cbl and α-tubulin