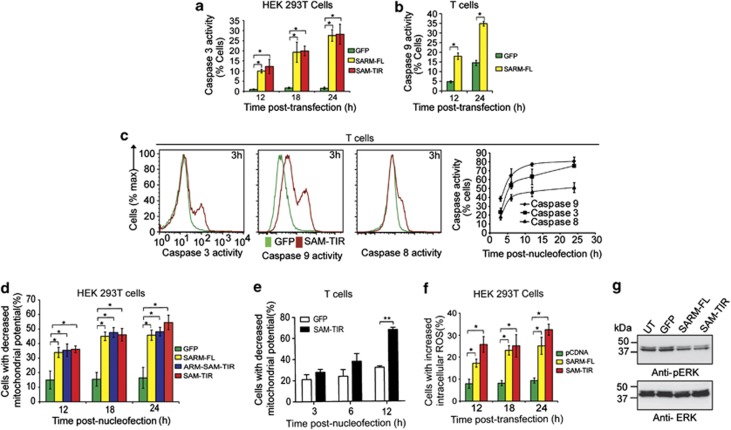
Figure 2.
SARM induces MPTP, generates ROS and inhibits ERK phosphorylation. (a) HEK 293T cells were transfected with 800 ng of SARM-FL/SAM–TIR/GFP plasmids. At 12, 18 and 24 h post-transfection, cells were loaded with fluorogenic caspase-3 substrate and the proportion of caspase-3 active cells was quantified. (b) Activated CD8 T cells expressing SARM was assessed for caspase-9 activities at 12 and 24 h post-nucleofection. (c) Activated primary CD8 T cells were nucleofected with SAM–TIR GFP/GFP alone and assayed for caspases-9, -3 and -8 activities. Representative flow cytometry data depict the overlay of caspase activity histogram of SAM–TIR- and GFP-transfected cells at 3 h (first 3 panels). The line graph (fourth panel) indicates the proportion of caspases-3, -9 and -8 active cells during the indicated time points of 3, 6, 12 and 24 h post-nucleofection. (d) HEK 293T cells transfected with SARM-FL/ARM–SAM–TIR/SAM–TIR/GFP alone were loaded with 20 nM of TMRE dye at 12, 18 and 24 h post-transfection. Decrease in mitochondrial transmembrane potential (ΔΨm) was computed based on TMRE fluorescence for each of the constructs. (e) Activated primary CD8 T cells were nucleofected with SAM–TIR/GFP and ΔΨm was quantified as above. (f) Intracellular ROS was detected in HEK 293T cells transfected with SARM-FL/SAM–TIR, using CM-H2DCFDA, at 12, 18 and 24 h post-transfection. The proportion of cells with increased intracellular ROS were calculated and plotted. (g) Cell lysates were prepared from SARM-FL-, SAM–TIR-, GFP-transfected and control untransfected (UT) HEK 293T cells, and immunoblotted with anti-pERK. ERK was used as the loading control. UT and GFP transfection did not affect ERK phosphorylation. Data in a–f represent means±S.D. of at least three independent experiments. *P<0.0005; **P<0.008