Figure 7.
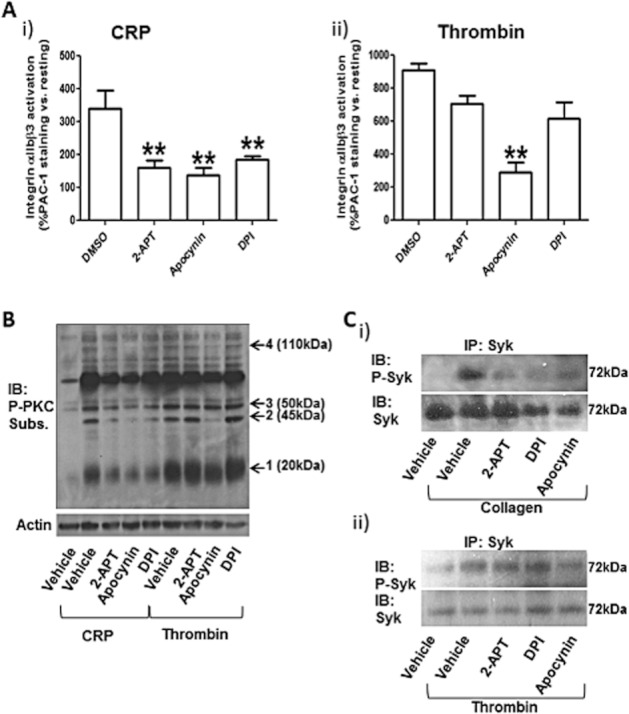
2-APT inhibits GPVI signalling. Integrin αIIbβ3 activation in response to 5 μg·mL−1 CRP (i) or 0.1 unit·mL−1 thrombin (ii) was monitored by flow cytometry using an activation-dependent FITC-conjugated antibody (PAC1) (A). Values are % fold increase over resting platelet (no stimulation) and are means ± SEM (n = 5). **P < 0.01; one-way anova with Bonferroni post test; n = 6 In (B), classical PKC isoform (cPKC) activation was tested by phospho-specific immunoblotting. cPKC-specific phosphorylation profiles showing the number and levels of proteins phosphorylated by these kinases are shown. Platelet activation was obtained by either CRP (5 μg·mL−1) or thrombin (0.1 unit·mL−1) following preincubation for 5 min with vehicle solution (0.1% v/v DMSO), 0.5 μM 2-APT, 0.5 mM apocynin or 100 μM DPI. Arrows numbered 1 to 4 indicate the bands analysed by densitometry in Supporting Information Figure S1. The results are representative of six independent experiments. In (C), Syk activation was also assessed by phospho-specific immunoblotting. Following treatment with NOX inhibitors at the same concentrations indicated above for 5 min, platelet stimulation was obtained with 10 μg·mL−1 collagen (i) or 0.1 unit·mL−1 thrombin (ii) in the presence of 1 mM EGTA for 5 min in continuous stirring (700 r.p.m.). Platelet lysis was performed in MCL buffer, and Syk was immunoprecipitated with 2 μg·mL−1 anti-Syk antibody in the presence of Protein A/G Plus agarose. The immunoprecipitates were resolved by SDS-PAGE and immunostained with anti-phospho-Syk (Tyr525/526) and anti-Syk antibodies. The results are representative of five independent experiments. IB, immunoblotting; IP, immunoprecipitation; Subs., substrate.
