Figure 2.
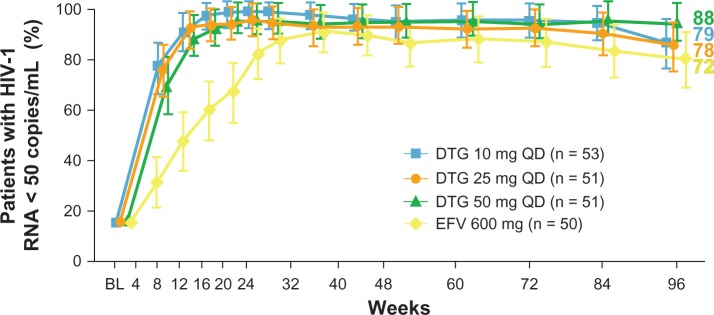
Percentage of subjects reaching human immunodeficiency virus type-1 RNA levels < 50 copies/mL at week 96 in the SPRING-1 trial.
Notes: Comparison between different dolutegravir doses and efavirenz 600 mg. The number of subjects enrolled in the study arms is shown in parentheses. Reprinted from The Lancet, 12(2), van Lunzen J, Maggiolo F, Arribas JR, et al. Once daily dolutegravir (S/GSK1349572) in combination therapy in antiretroviral-naive adults with HIV: planned interim 48 week results from SPRING-1, a dose-ranging, randomised, phase 2b trial. Lancet Infect Dis. 2012;12(2):111–118. Copyright 2012, with permission from Elsevier.45
Abbreviations: DTG, dolutegravir; EFV, efavirenz.
