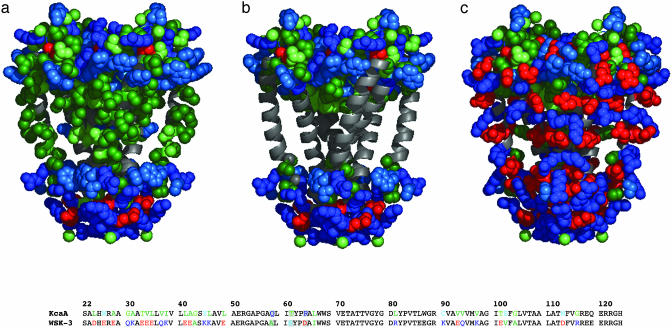
Fig. 2.
Depiction of KcsA and WSK-3. Only the side chains of outer helices (22–71), inner helices (89–124), and cytoplasmic and extracellular residues are shown. Side chains are colored based on frequency of occurrence in the apolar section of the lipid bilayer beginning with most probable: Ala/Ile/Leu/Val (dark green), Gly/Met/Thr (light green), and Pro/Ser/Trp/Tyr (light blue). Lys/Arg/Gln (dark blue), Asp/Glu (red). (a) KcsA. Solvent-exposed residues of KcsA allowed to vary in the design are depicted along the inner and outer helices (light green, dark green). Also shown are the cytoplasmic and extracellular residues, unchanged in our design. (b) KcsA structure with side chains of mutated residues removed. Extracellular and cytoplasmic residues held constant from KcsA to WSK are rendered. Buried residues within the interior of the structure are not shown. (c) WSK-3. Sequences of KcsA and WSK-3 are also shown, where colored residues were mutated, whereas those in black were not. Mutations to tKcsA are shown in a gray box [made by using pymol (DeLano Scientific, San Carlos, CA).