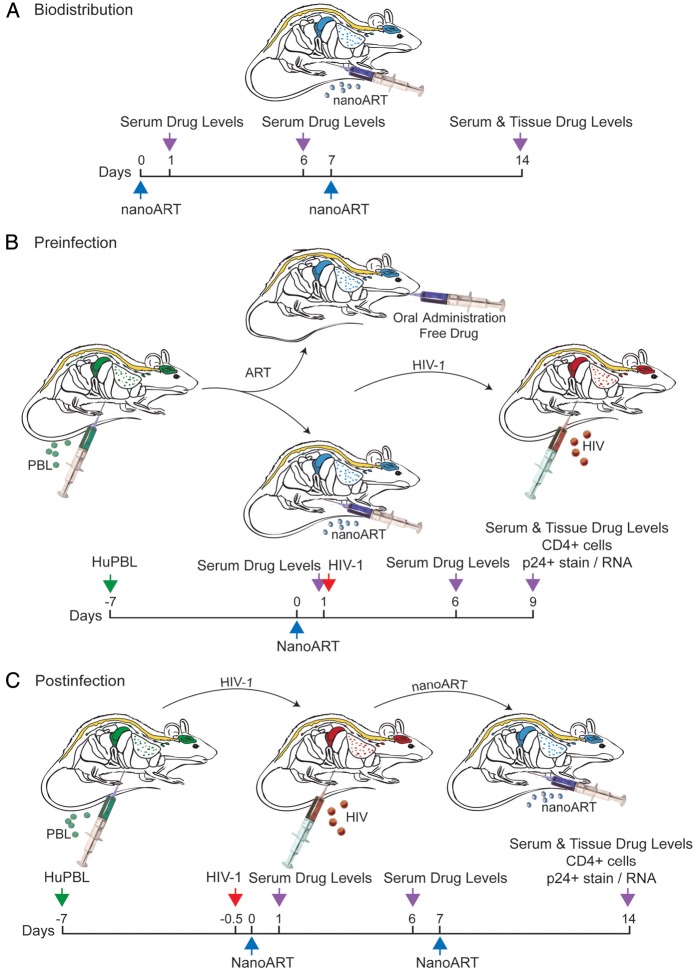
Figure 1.
Experimental protocols used in studies of nanoformulated antiretroviral therapy (nanoART) efficacy and toxicology profiles. A, Treatment paradigm to determine pharmacokinetics and tissue distribution of nanoART (M2001 [atazanavir, ATV] + M3001 [ritonavir, RTV]; 80, 150, or 250 mg/kg each drug) by subcutaneous administration to NSG mice on days 0 and 7. B, Treatment paradigm to determine preinfection antiviral activity of nanoART (H2001 [ATV] + H3001 [RTV]; 250 mg/kg each drug) by subcutaneous administration on day 0 to human peripheral blood lymphocyte (huPBL)–reconstituted NSG mice 24 h prior to human immunodeficiency virus type 1 (HIV-1) infection. C, Treatment paradigm to determine antiviral activity of nanoART (H2001 + H3001, 250 mg/kg each drug or H2001 + H3001 + H4001 [efavirenz, EFV], 100 mg/kg each drug) by subcutaneous administration on days 0 and 7 to PBL-reconstituted, HIV-1–infected NSG mice.