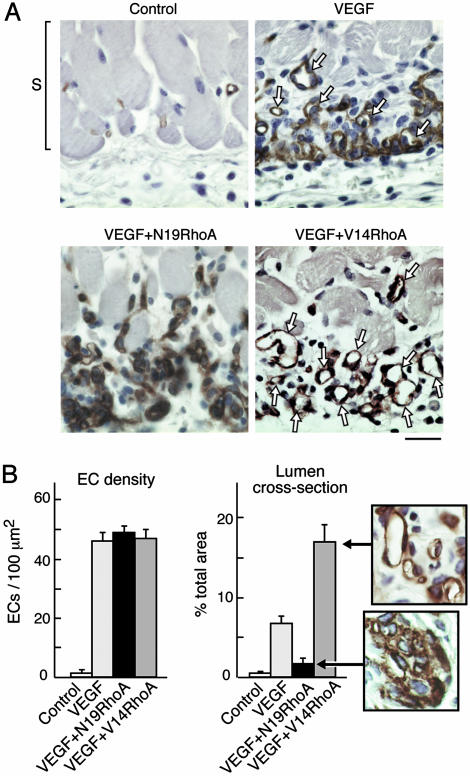
Fig. 2.
RhoA mutants differentially regulate assembly of ECs into new blood vessels in vivo. (A) Skin specimens (day 6, as in Fig. 1) were fixed and embedded in paraffin, and ECs were stained for CD31. Staining of new ECs (brown), immediately below the smooth muscle cell layer (S), and at the interface between the host and the angiogenic stimulus, was strong in all experimental groups except the negative controls. New ECs of the VEGF group were only partially integrated into blood vessels with lumens (arrows). In contrast, new ECs of the V14RhoA plus VEGF group were highly integrated into new blood vessels with lumens (arrows), whereas new blood vessels with lumens were rare in the N19 RhoA plus VEGF group. Photographs are representative examples of results consistently obtained in two separate experiments; n > 12 for each group; bar = 25 μm. (B) Magnified views illustrate strong organization of ECs into blood vessels with lumens in the VEGF plus V14RhoA group and, in contrast, the failure of ECs in VEGF plus N19RhoA group to organize detectably. Such images were used for morphometric quantification of vascular cross-sectional area attributable to blood vessels with functional lumens (Right) and morphometric quantification of EC density (Left). Error bars = standard errors; n > 20 for each group. We observed no significant differences in EC density between the N19RhoA and V14RhoA groups; however, differences in cross-sectional area of blood vessel lumens were highly significant (P < 0.001).