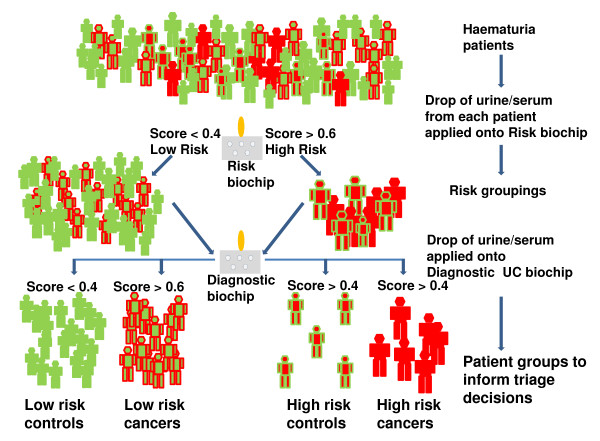
Figure 4.
Translation of classifiers into biochip format for risk stratification of hematuria patients. In the future when a patient with hematuria presents in primary care, their urine and serum samples could be sent for evaluation using biochips (grey oblongs). One biochip could be created for risk stratification and one biochip for the diagnosis of UC. Each biochip would be formatted with approximately six antibody spots, referred to as test regions. The underlying concept of these biochips is based on procedures similar to an ELISA, that is, light readings are generated from each test region which are proportional to the bound protein that is present in each patient's sample. Computer software would generate a score between 0 and 1 for each patient's sample. For the risk biochip, scores <0.4 would suggest a low risk of serious disease, while scores >0.6 would suggest a high risk of serious disease. The patient could then be designated low-risk (green) or high-risk (red) risk. Patients would then be screened using a second biochip, this time a UC diagnostic biochip. Similarly, scores <0.4 from the UC diagnostic biochip would suggest that it was unlikely that the patient would have bladder cancer while scores >0.6 would suggest that the patient requires further investigations to check for the presence of UC. The scores from both biochips would be interpreted alongside clinical parameters. The patient's clinician would then make a triage decision for that patient which would be informed by the biochip scores. For example, a high-risk UC patient (all red) could obtain a score >0.6 on the scale ranging from 0 to 1 for both biochips and likewise a low-risk control could receive a score <0.4 for both biochips. ELISA, enzyme-linked immunosorbent assay; UC, urothelial cancer.