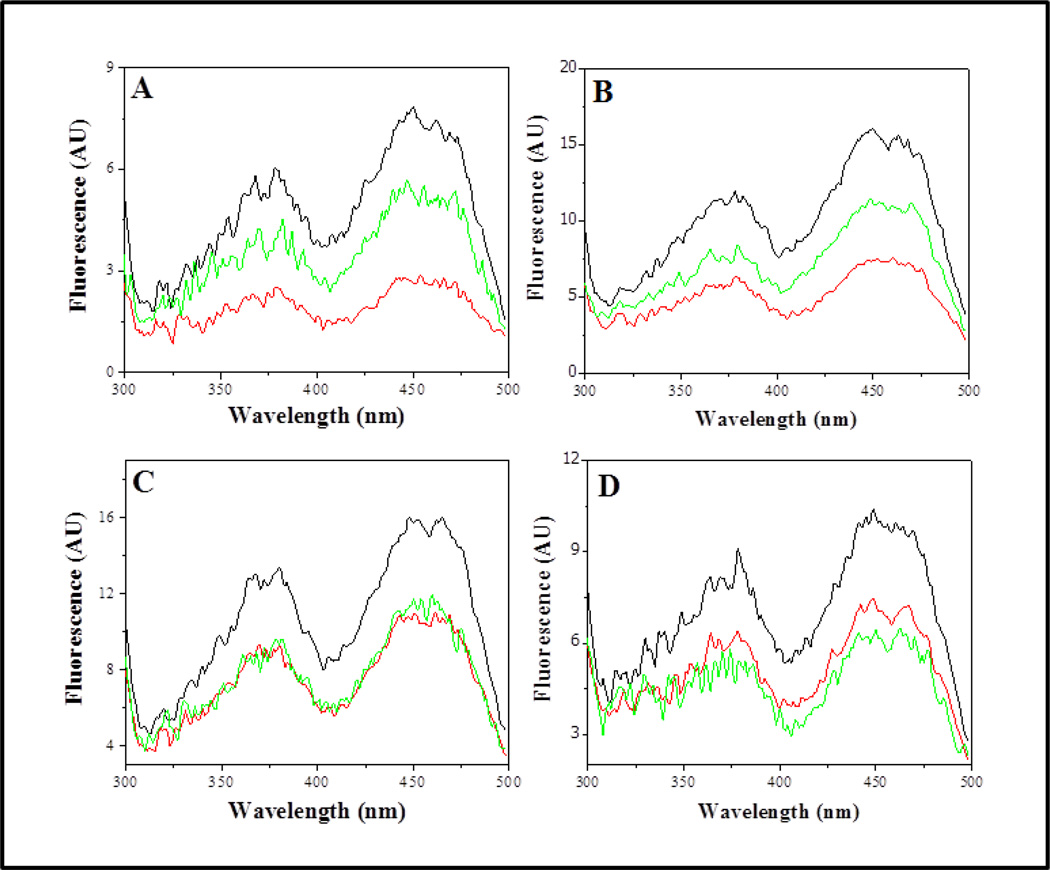
Figure 6. Fluorescence excitation spectra of purified wild type and mutant C. maquilingensis SQR.
Excitation spectra are shown for (A) wild type, (B) M380N, (C) L379D and (D) L379N SQR proteins. Spectra were recorded prior to substrate addition, (black lines); 20 min after addition of 150 µM sulfide (red lines); 20 min after addition of 100 µM decylubiquinone (green lines). All experiments were performed at room temperature, resulting in slow oxidation and reduction of the FAD. Reoxidation of the FAD upon addition of decylubiquinone is not observed for the L379D and L379N mutants. The protein concentration was 1 mg ml−1 (22 µM protein; ~11µM FAD). The emission was monitored at 520 nm. Data are representative of results of at least three separate experiments. AU, arbitrary units.