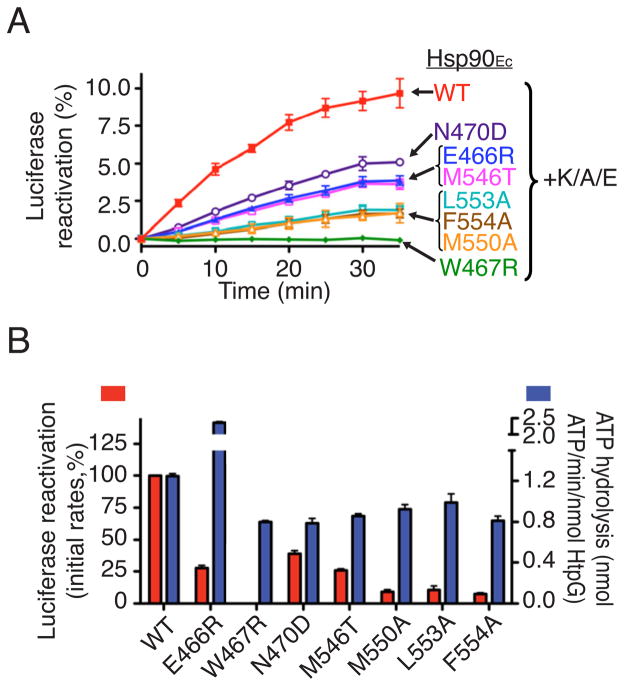
Figure 3. The Hsp90Ec mutant proteins exhibit defective chaperone activity in vitro.
(A) Reactivation of heat-denatured luciferase by Hsp90Ec wild type or mutants in conjunction with DnaK, CbpA and GrpE (K/A/E) was measured over time. The value obtained with K/A/E alone was subtracted. It was previously shown that only the soluble fraction of the denatured luciferase is reactivated by Hsp90Ec and the DnaK system and that this soluble fraction corresponds to about 20% of the total luciferase (Genest et al., 2011). Thus Hsp90Ec wild type in combination with the DnaK system reactivates about 50% of the soluble inactive luciferase in this experiment.
(B) Initial linear rates of luciferase reactivation (red) by Hsp90Ec wild type or mutants in the presence of K/A/E were calculated from Figure 3A and the rate of wild type reactivation set to 100%. The ATPase activity of Hsp90Ec wild type or mutants was measured and is represented in blue.
In (A) and (B), data from three replicates are presented as mean ± SEM.
See also Figure S3.