Figure 5. Co-translational interaction of nascent chains facilitates the elongation of polypeptides.
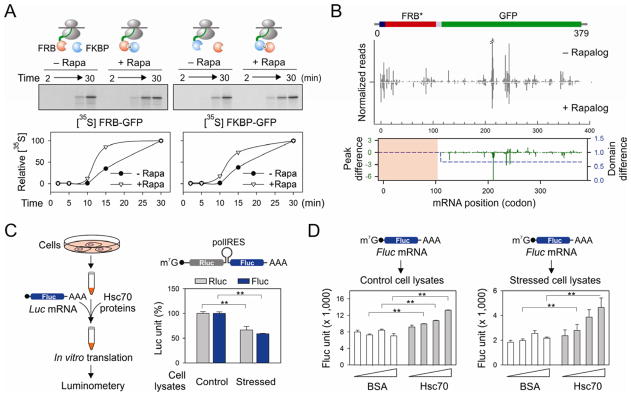
(A) Effects of FKBP (blue ball) on the in vitro translation of FRB-GFP (red ball) in the absence or presence of 1 μM rapamycin (left panel). The right panel shows the effects of FRB (red ball) on the in vitro translation of FKBP-GFP (blue ball) in the absence or presence of 1 μM rapamycin. Autoradiography of full length GFP fusion protein is quantitated and plotted as a function of time.
(B) HEK293 expressing FRB*-GFP was transfected with the plasmid encoding FKBP. Cells were pre-treated with 1 μM rapalog for 60 min before polysome profiling. The RPF density profiles are shown for the transgene FRB*-GFP with and without rapalog treatment. The RPF reads density is normalized based on the FRB* domain. The average change of RPF density over the entire GFP region (blue dot line) and single codon change (green line) are plotted together (Wilcoxon signed-rank test, p-value = 3 × 10−4). See also Figure S7
(C) Schematic of experimental design using recombinant Hsc70 protein to restore translation efficiency using an in vitro translation system programmed from cells with or without proteotoxic stress. The right panel shows the relative translation efficiency of a synthesized bicistronic mRNA containing a polio IRES element between Rluc and Fluc. Error bar: SEM. **, p < 0.001.
(D) The in vitro translation system as (C) was used to translate a synthesized Fluc mRNA in the absence or presence of recombinant Hsc70. Error bar: SEM. **, p < 0.01.
