Figure 2.
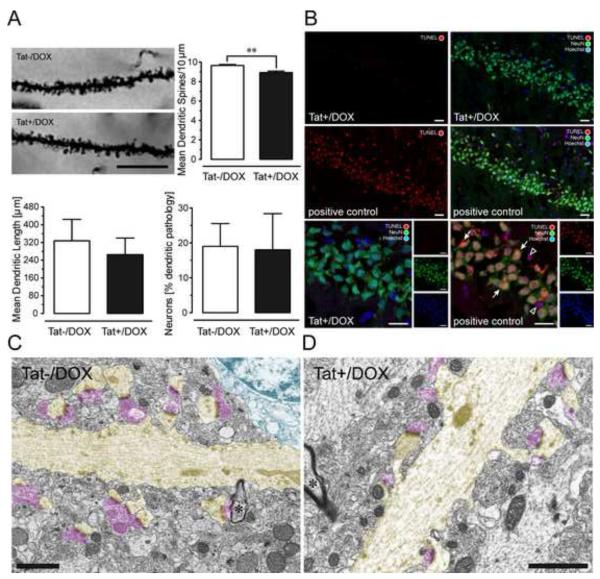
(A) Effects of Tat induction on number of dendritic spines, dendritic length, and morphology on pyramidal neurons in the stratum radiatum of the hippocampal field CA1 (2-3-mo old mice). Spine density was assessed in Golgi-Kopsch impregnated neurons. Scale bars = 10 μm. Number of dendritic spines were counted on apical dendrites, recorded as the mean number of spines per 10 μm dendrite length, averaged for each animal, and reported as changes in the mean spine density (number of spines/10 μm). Significant decreases in total spine density were seen in the inducible (Tat+/DOX, n = 6) mice following induction with DOX compared to control (Tat−/DOX, n = 6) mice [**t(10) = 3.89, p < 0.01]. Length of apical dendrites was measured, since afferent synapses arriving from the Schaffer collaterals (see LTP studies below) preferentially ramify on apical dendrites within the stratum radiatum. For morphological assessment, each neuron was categorized either as having apical dendrites with an entirely normal morphology, or having an abnormal morphology, with one or more dendrites that displayed aberrant features, such as beading and fragmentation along proximal and/or distal segments [see also 51]. The proportion of neurons that possessed one or more dendrites with abnormal morphology was counted and reported as a percentage of total neurons examined. No effects were noted on dendritic length or dendritic morphology. Data are represented as mean (± SEM). (B) Effects of Tat induction on TUNEL detection in NeuN immunoreactive pyramidal neurons in the hippocampal field CA1. TUNEL was employed to detect in situ DNA fragmentation using the In Situ Cell Death Detection Kit, TMR red (Roche Applied Science, Indianapolis, IN). Positive control tissue for the TUNEL reaction was incubated with micrococcal nuclease or recombinant DNase I for 10 min at +15 to 25°C to induce DNA strand breaks prior to the TUNEL labeling procedure. TUNEL detection was almost never detected in the Tat+/DOX group (n = 4), whereas most Neu-N(+) and NeuN(−) TUNEL-positive cells were abundant in the positive control. The positive control indicates DNA fragmentation for NeuN immunoreactive neurons in the CA1 layer (arrow) as well as in cells that were not NeuN immunostained, likely representing glia (open arrowhead); DOX: doxycycline. (C-D) Electron micrographs of dendrites and associated postsynaptic spines (highlighted in yellow) in the CA1 region of the hippocampus of Tat+/DOX mice. Dendrites are distinguished from myelinated axons by their lighter cytoplasm, abundant microtubules, thicker diameter, and dendritic spines. The asterisk indicates a myelinated axon (*). Note continuity of dendrites with their spinous processes. Despite loss of function shown in Fig. 4 and changes in synaptic proteins shown in Fig. 3, the ultrastructure looks relatively normal in Tat+/DOX mice (D) comparable to Tat−/DOX mice (C). Both asymmetric synapses contacting dendritic spines, as well as the symmetric synapses on the dendritic shaft appeared qualitatively normal. Pre-synaptic elements highlighted in pink; Scale bar = 1 μm.