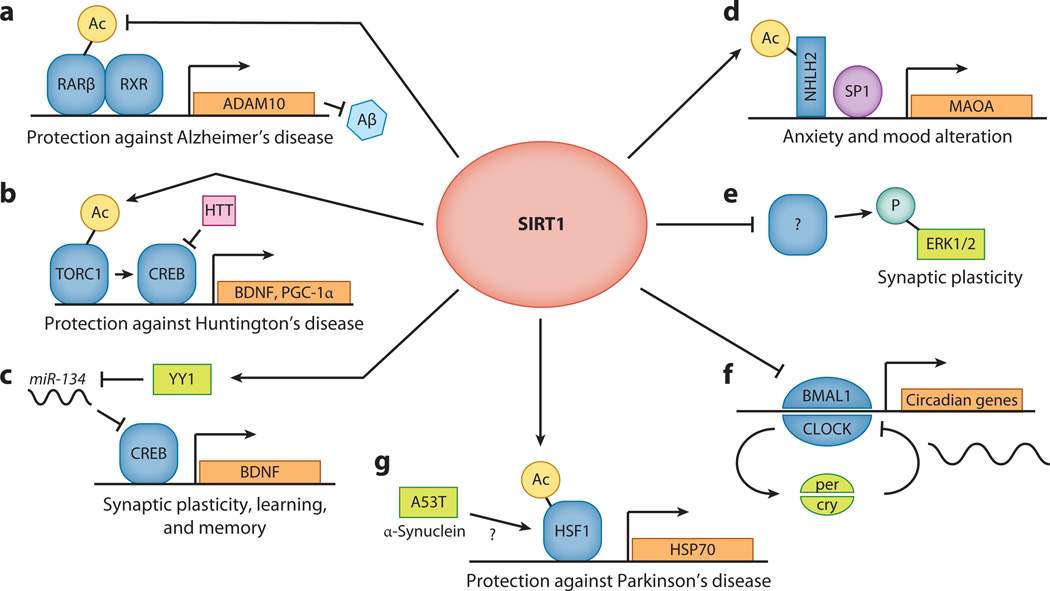
Figure 6.
SIRT1 in the brain is involved in a number of processes that affect neuronal health, neurogenesis, and behavior. (a) SIRT1 can deacetylate retinoic acid receptor β (RARβ), which causes the heterodimerization of RARβ with retinoid X receptor (RXR) on the promoter of ADAM10, which encodes α-secretase. Increases in the abundance and activity of ADAM10 direct amyloid precursor peptide (APP) processing toward the α-secretase pathway. This in turn leads to less β-secretase cleavage of APP and thus less Aβ production, which is protective against Alzheimer’s disease. (b) SIRT1 deacetylates and activates TORC1 by promoting its dephosphorylation and its interaction with CREB. Brain-derived neurotrophic factor (BDNF) and PGC-1α are key targets of SIRT1 and TORC1 transcriptional activity. Both targets counteract the toxic effects of the Huntingtin protein and protect against Huntington’s disease. (c) SIRT1 limits miR-134 expression via a repressor complex containing the transcription factor YY1. If SIRT1 is downregulated and miR-134 expression is increased, CREB and BDNF are downregulated, thereby impairing synaptic plasticity. (d) SIRT1 deacetylates and thus activates the neuronal helix-loop-helix 2 (NHLH2) transcription factor, which in conjunction with SP1 controls the transcription of monoamine oxidase A (MAOA). MAOA activity alters the abundance of neurotransmitters, such as serotonin and noradrenaline, which in turn influence an animal’s mood and behavior. (e) SIRT1 knockout results in decreased extracellular signal–regulated kinase 1/2 (ERK1/2) phosphorylation and in altered expression of hippocampal genes involved in synaptic function. SIRT1 knockout animals have compromised cognitive functions, reinforcing the notion that SIRT1 is indispensable for normal learning, memory, and synaptic plasticity. (f) SIRT1 binds the CLOCK-BMAL1 complex in a circadian manner and promotes the deacetylation and degradation of cytochrome PER2. The PER2/CRY complex suppresses the activity of CLOCK/BMAL1, forming a negative feedback loop that results in periodic oscillation of the abundance of these molecules. SIRT1 is required for normal circadian transcription of core clock genes in the liver and connects cellular metabolism to the circadian rhythm. (g) SIRT1 protects against Parkinson’s disease by deacetylating heat shock factor 1 (HSF1) to increase activation of its targets, such as heat shock protein 70 (HSP70). Interestingly, the induction of HSF1 activity requires the overexpression of both SIRT1 and the A53T disease gene.