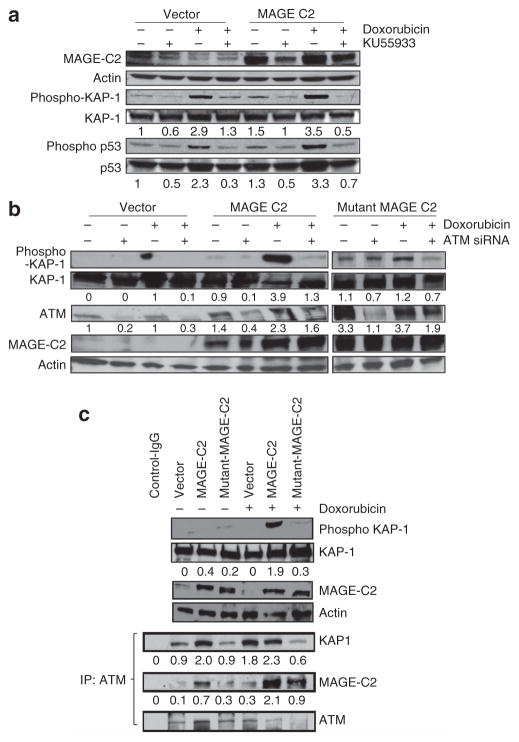
Figure 5. Melanoma-associated antigen-encoding (MAGE)-C2 binds to KAP-1 and induces ataxia-telangiectasia–mutated (ATM) kinase–dependent phosphorylation of KAP1-Ser824.
(a) HEK293T cells, ectopically expressing MAGE-C2, were treated with doxorubicin and KU55933, a specific ATM kinase inhibitor. MAGE-C2-induced phosphorylation of KAP1 was blocked by the ATM kinase inhibitor KU55933. KU55933 treatment also causes reduction in phosphorylation of p53, another substrate of ATM kinase, which confirms KU55933 activity. (b) HEK293T cells, with ectopic expression of wild-type MAGE-C2 or mutant MAGE-C2L152A L153A (unable to bind KAP1), were transfected with ATM kinase small interfering RNA (siRNA) and treated with doxorubicin. Immunoblotting shows that ATM siRNA inhibits MAGE-C2-induced as well as DNA damage–induced phosphorylation of KAP1-Ser824, confirming the effect of KU55933. (c) Immunoblotting shows that wild-type MAGE-C2, but not mutant MAGE-C2L152A L153A, increases KAP1-Ser824 phosphorylation, in response to doxorubicin. Wild-type MAGE-C2, in the presence of doxorubicin treatment, increases co-precipitation of ATM kinase and KAP-1. Unable to bind to KAP-1, mutant MAGE-C2L152A L153A has no effect on ATM and KAP-1 co-precipitation. IP, immunoprecipitation.