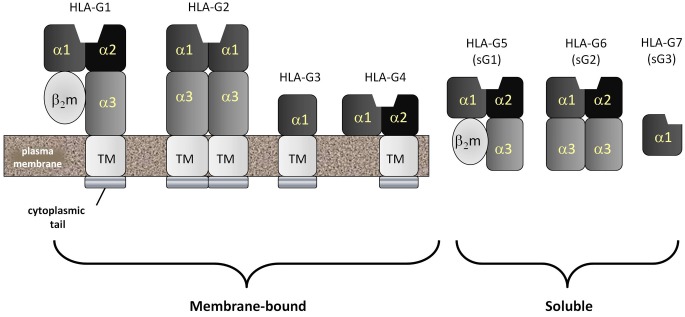
Figure 3.
HLA-G: the most common form of the non-classical MHC class Ib molecule, HLA-G, mimics HLA-A and -B in structure and is called HLA-G1. HLA-A, -B and -G1 are all homodimers of an MHC class I heavy chain comprised of five domains and a stabilizing second molecule, beta-2 microglobulin (β2m). The MHC class I heavy chain consists of an α1 and α2 domain (forming the antigenic peptide-binding groove), an α3 domain, a transmembrane domain and a cytopalasmic tail. Unlike classical MHC class I molecules, the cytoplasmic tail of HLA-G is very short, containing only six amino acids. Also unlike classical MHC class Ia molecules, HLA-G can be detected as several spliced variants. The most common of these are the membrane-bound HLA-G1, -G2, -G3 and -G4 and the soluble HLA-G5, -G6 and -G7. Soluble forms have lost their transmembrane segments and cytoplasmic tails during splicing.