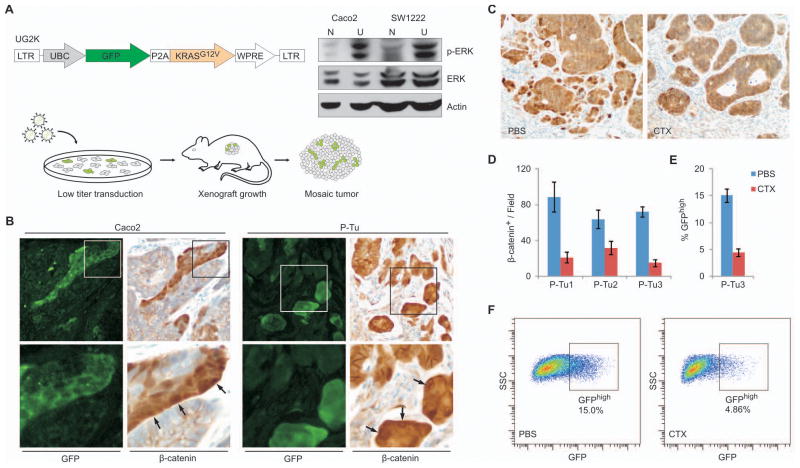
Figure 4. KRAS activation increases, and interference with EGFR signaling decreases the WNT-active cell fraction in Caco2 and primary tumor xenografts.
(A) Vector schema: Lentiviral vector UG2K for Ubiquitin-C promoter (UBC) driven overexpression of mutant KRASG12V together with GFP, separated by a P2A cleavage peptide. Right panel: Immunoblot showing increased phospho-ERK (p-ERK) and unchanged total-ERK (ERK) expression in UG2K-transduced (U), serum starved cells compared to native (N) Caco2 and SW1222 cells. Actin served as a loading control. Bottom: Experimental scheme to generate colon cancer xenografts with mosaic UG2K transduction. Colon cancer cell lines and primary tumor xenografts were exposed for short periods to low titers of UG2K lentivirus and injected subcutaneously into NOD/SCID mice for tumor formation. (B) Sections of mosaic UG2K-transduced tumor xenografts double stained for GFP and β-catenin, showing increased nuclear β-catenin in GFP-expressing tumor cells (arrows). Images in the lower panels show higher magnifications of areas boxed in the upper panels. (C–D) Representative β-catenin immune staining of a primary colon cancer xenograft (C) and quantitation of the β-catenin positive cell fraction in 3 primary colon cancer xenografts (D) treated in vivo with cetuximab (CTX) or PBS. (E–F) Reduced GFPhigh tumor cell fraction in TOP-GFP.mC transduced primary colon cancer xenografts following cetuximab (CTX) treatment, as measured by flow cytometry of disaggregated tumor cells (F). Error bars indicate the standard error of the mean from 3 experiments.