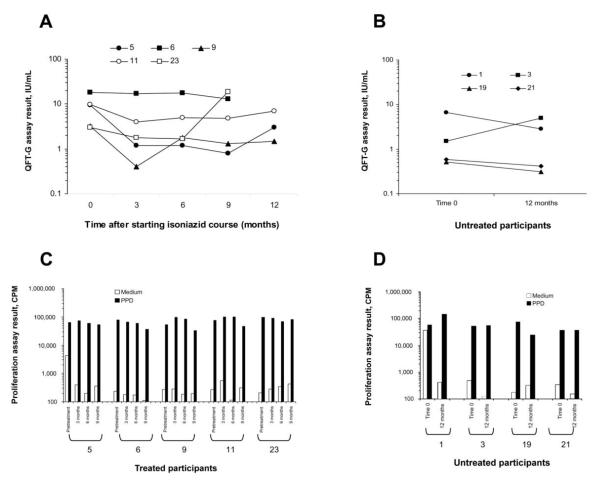
FIGURE.
Comparison of results of serial QuantiFERON-TB Gold (QFT-G) assays and proliferation assays for participants who received isoniazid treatment and those who did not receive isoniazid treatment. A, QFT-G results for the 5 treated participants (participants 5, 6, 9, 11, and 23) who completed a full 9-month course of isoniazid treatment and the entire serial testing protocol (testing at baseline and at 3, 6, and 9 months; 3 of 5 participants also had tests performed at 12 months). B, QFT-G results for the 4 untreated control subjects (participants 1, 3, 19, and 21) who had tests performed at baseline and at 12 months. For both groups, results of the QFT-G assays for each participant at the given time point are expressed as the higher of the 2 possible test results (early secretory antigenic target–6-nil or culture filtrate protein–10-nil), to depict the maximum response in the assay at the given time point. C and D, Responses of the 5 participants who received treatment (C) and the 4 participants who did not receive treatment (D) in extended (6-day) cellular proliferation assays in which peripheral blood mononuclear cells obtained from the participants at each time point (at baseline and at 3, 6, and 9 months for participants who received treatment and at baseline and at 12 months for untreated participants) were stimulated with purified protein derivative. Assay results are expressed as counts per minute (CPM) in wells containing medium alone or medium and purified protein derivative.