Abstract
Q fever is a zoonotic disease caused by infection with the bacterium Coxiella burnetii. Infection with C. burnetii results in humoral and cellular immune responses, both of which are thought to contribute to protection against subsequent infection. Whole-cell formalin-inactivated vaccines have also been shown to induce both humoral and cellular immunity and provide protection. Whether measurement of cellular or humoral immunity is a better indicator of immune protection is not known, and the duration of immunity induced by natural infection or vaccination is also poorly understood. To better understand the measurement and duration of C. burnetii immunity, 16 people vaccinated against Q fever (0.2 to 10.3 years before analysis) and 29 controls with a low risk of Q fever exposure were tested for immune responses to C. burnetii by an indirect fluorescent-antibody test (IFA) to measure circulating antibody and by a gamma interferon release assay (IGRA) to measure cellular immunity. Among vaccinated subjects, the IFA detected antibodies in 13/16, and the IGRA also detected positive responses in 13/16. All of the vaccinated subjects had a positive response in at least one of the assays, whereas 8/29 control subjects were positive in at least one assay. There was not a correlation between time since vaccination and responses in these assays. These results show that IFA and IGRA perform similarly in detection of C. burnetii immune responses and that Q fever vaccination establishes long-lived immune responses to C. burnetii.
INTRODUCTION
Coxiella burnetii infection of humans causes Q fever, a flu-like illness whose symptoms typically include fever, headache, and myalgia (1). In some cases, pneumonia and/or hepatitis can be present. Most patients resolve the infection and are immune to further C. burnetii infections, but a minority of patients are unable to clear the bacteria and develop a chronic infection that most often presents as culture-negative endocarditis (2). C. burnetii is a Gram-negative bacterium that is typically transmitted by inhalation of aerosols that contain the bacteria (3). Once the organism is in the lungs, cells of the monocyte/macrophage lineage are infected (4). Infections can be initiated with small numbers of organisms, and the bacteria are slow growing in vivo. Humans therefore have a dose-dependent incubation period of 1 to 3 weeks before the onset of symptoms (1). At the time of symptom onset, C. burnetii organisms are often detectable in blood and serum (5).
Antibody responses develop 7 to 14 days after the onset of symptoms, with IgG antibody appearing shortly after IgM (1). Once IgG antibodies are present, C. burnetii quickly becomes undetectable in the blood (5). The mechanism by which C. burnetii is cleared from the blood is not known, but the timing correlates well with the development of immune responses (5). Although recent reports have suggested that C. burnetii DNA and antigen can be detected years after an acute infection, viable organisms are thought to be eliminated more quickly (6, 7). However, the time required for complete clearance of viable C. burnetii in humans is not known. Cellular immune responses are thought to be initiated in humans with kinetics similar to those of the antibody response, but this has not been studied extensively (8).
Mouse models of C. burnetii infection have been used to demonstrate that both CD4 and CD8 T cells are needed for clearance of the agent, with B cells playing a supporting role (9). Both serum and splenocytes from immune mice can transfer significant protection against C. burnetii to naïve mice, but only transfer of immune splenocytes can confer protection on SCID mice (10). These studies have suggested that both T and B cell responses play a significant role in protective immunity in humans.
The only currently commercially available human vaccine against Q fever is Q-Vax, a whole-cell formalin-inactivated preparation of the phase 1 Henzerling strain of C. burnetii. This vaccine is licensed for use only in Australia, where it is given primarily to farmers, abattoir workers, and laboratorians. The vaccine has been demonstrated to be highly effective and has a strong safety record (11). The vaccine cannot be given to persons already immune to C. burnetii, as this can cause a severe adverse reaction at the injection site (11). Because of this, potential vaccinees have to be carefully screened both for anti-Coxiella antibodies and by a skin test to identify potentially adverse responses. Both of these tests need to be negative before vaccination is advised (11). Previously, a Q fever vaccine based on a formalin-inactivated form of the phase 1 Henzerling strain of C. burnetii was available from the Special Immunizations Program of the U.S. Army Medical Research Institute for Infectious Diseases (USAMRIID) as an investigational new drug (IND). This vaccine is not currently available and has been placed on hold due to potency issues with the skin test (12).
The longevity of the protective immune response against C. burnetii provided by either natural infection or vaccination has not been well defined. The most common approach to evaluate immunity has been the measurement of the levels of serum antibody against C. burnetii. A study from the Netherlands that followed serology in a large group of naturally infected persons observed during that country's 2007-2011 Q fever epidemic found that IgG phase 2 serum antibody peaked at a mean of 53 days after symptom onset and then declined slowly, with the half-time of the antibody decay rate being 318 days (13). Thus, greater than 20% of acute Q fever patients could become seronegative 3 to 4 years after having the disease. Indeed, an analysis of Q fever patients in Australia 6 years after an outbreak found that 7/38 (18.4%) had become seronegative (7). A study of antibody responses in people vaccinated against Q fever found that only 60% had positive titers 20 months after vaccination, whereas 90% had detectable cellular immune responses (11). These results suggest that measurement of cellular immunity should be considered an indicator of previous exposure to C. burnetii. However, detection of cellular immunity in vaccinated subjects has also been reported as variable (14).
The purpose of this study was to compare the abilities of antibody assays and measurement of T cell responses to detect immune responses to C. burnetii in vaccinated people. Because of access to a pool of subjects vaccinated at various times, this study also examined the longevity of these immune responses in Q fever vaccinees.
MATERIALS AND METHODS
Blood donors.
Vaccinated donors were recruited from the Centers for Disease Control and Prevention (CDC) campus in Atlanta, GA. Vaccinated donors provided their date of vaccination at the time of blood donation. Control donors were recruited from the Emory/CDC blood donor program. The control donors were considered to be at relatively low risk for Q fever exposure; farmers and veterinarians were excluded from this group. All donors gave informed consent before donation. The study was approved by the CDC Institutional Review Board (protocol no. 6019).
IFN-γ release assay (IGRA).
Heparinized whole blood was aliquoted into wells of a 24-well tissue culture plate at 1 ml per well. The wells were inoculated with either 20 μg/ml PHA-L (Sigma, St. Louis, MO), 9 μg/ml Nine Mile phase 1 C. burnetii antigen (see below), or nothing. Plates were then incubated for 22 h at 37°C and 5% CO2. After incubation, 200 μl plasma was removed from each well and analyzed for the presence of gamma interferon (IFN-γ) using a Bio-Plex Luminex 100 (Bio-Rad, Hercules, CA) with a human IFN-γ fluorokine MAP kit (R&D Systems, Minneapolis, MN).
Antigen preparation.
The antigen was a chloroform-methanol residue (CMR) of C. burnetii strain Nine Mile phase 1 (RSA493). The CMR preparation method was based on a published technique (15). The bacteria were grown in ACCM-2 axenic media at 2.5% O2 and 5% CO2. After 6 days of growth, cells were pelleted, washed with water, and then resuspended in 1% formaldehyde. The cells were then incubated at room temperature for 24 h. The fixed organisms were then washed 3 times in deionized water and lyophilized. The lyophilized C. burnetii organisms were refluxed with a 4:1 mixture of chloroform-methanol in a microcentrifuge tube at 70°C for 6 h. The resulting mixture was put through a Whatman no. 1 filter, and the residue on the filter was refluxed in 4:1 chloroform-methanol for another 6 h. After filtration, the residue was refluxed again, followed by filtration. The resulting residue was resuspended in phosphate-buffered saline (PBS) and used as the antigen. Protein concentration was determined using a bicinchoninic acid (BCA) protein assay (Bio-Rad). The CMR of C. burnetii has been reported to contain lipopolysaccharide (LPS), protein, and peptidoglycan (16).
IFA.
Antibodies to C. burnetii were measured by indirect fluorescent-antibody test (IFA). Heparinized whole blood was centrifuged at 800 × g, and plasma was removed. Wells on slides coated with either a Nine Mile phase 1 (RSA493) or Nine Mile phase 2 (RSA439) strain of C. burnetii were treated with titrations of plasma samples. Washed slides were treated with fluorescein isothiocyanate (FITC)-conjugated goat anti-human antibody, and binding was visualized using a fluorescence microscope. The titers reported are the greatest dilution of plasma that resulted in unambiguous antibody binding.
Statistics.
Student's t 95% confidence intervals (CIs) were determined for mean IgG log2 titer and IFN-γ values for both vaccinated and control groups, and geometric mean titers (GMTs) and associated CIs were calculated from these by exponentiation as appropriate. Comparison of log2-GMTs and IFN-γ means were made using Student's t 95% CI for the differences between the groups; the GMT (and CI) comparison was expressed directly as a ratio by exponentiation of this result. A cutoff for positivity for the IFN-γ values was derived using receiver operating characteristic (ROC) curve analysis, where the cutoff was determined from the ROC curve by choosing the IFN-γ value that yielded equal empirical sensitivity and specificity. Linear models were used to evaluate whether there was a statistically significant decline in IgG titer and IFN-γ responses with time (in years since vaccination) by estimating the coefficient (and 95% CI) for the time since vaccination, along with an analysis of variance (ANOVA) test that the coefficient was positive.
RESULTS
Since 2002, the CDC has been providing Q fever vaccination to workers whose job duties include a potential for exposure to C. burnetii. Between 2002 and 2004, these workers were vaccinated using the IND vaccine from the special-immunization program at USAMRIID. Between 2007 and the present, workers have been vaccinated with Q-Vax in Australia. Personnel that received vaccination from these 2 sources were offered an opportunity to participate in this study, and a total of 16 Q fever-vaccinated people enrolled. Eleven of the volunteers received the Australian Q-Vax vaccination, and 5 enrollees received the IND vaccination from USAMRIID. Twenty-nine volunteer donors that were considered to be at low risk for previous exposure to Q fever were also enrolled as a control group.
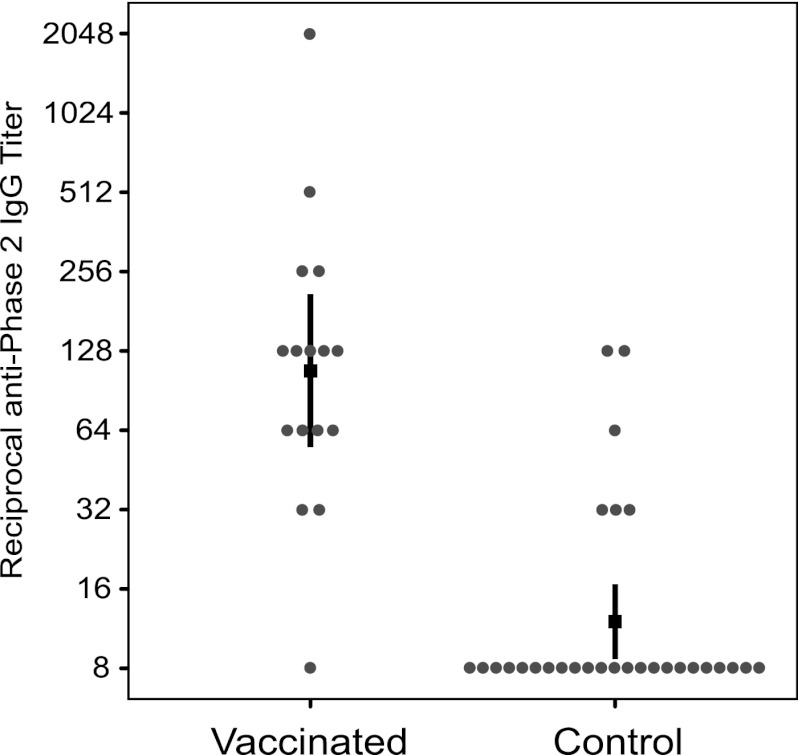
The humoral and cellular immune responses in the participants were evaluated. Humoral immunity was measured by determination of plasma IgG antibody levels using IFA. Individual IgG titers against phase 2 antigen in the vaccinated group varied, with one donor having a titer of less than 1:16 and the highest titer being 1:2,048, with the other titers distributed between these extremes (Fig. 1). Titers in the control group were mostly less than 1:16, although titers of 1:32, 1:64, and 1:128 were also observed. Assigning a titer of 1:8 for a negative result, the GMT of IgG against phase 2 Nine Mile C. burnetii antigen in the vaccinated group was 1:108 (95% CI, 1:55.2 to 1:209.8), whereas the GMT in the control group was 1:12.0 (95% CI, 1:8.7 to 1:16.6) (Fig. 1). The two GMTs were significantly different, with a ratio of 1:9.0 (95% CI, 1:4.3 to 1:18.5). To determine a cutoff for a positive measurement, potential cutoffs of 1:32, 1:64, and 1:128 were considered. The cutoff of 1:64 gave results where sensitivity (81.25%) and specificity (89.66%) were most closely matched; this cutoff therefore resulted in IFA calling 13/16 (81.25%) vaccinated subjects positive and 3/29 (10.3%) of the control subjects positive.
Fig 1.
IgG antibody titers in Q fever-vaccinated and control subjects. Titers of IgG to Nine Mile phase 2 antigen were measured by IFA. The titers for 16 Q fever-vaccinated subjects and 29 control subjects are shown. The black square indicates the geometric mean titer, and the vertical lines show the 95% confidence intervals.
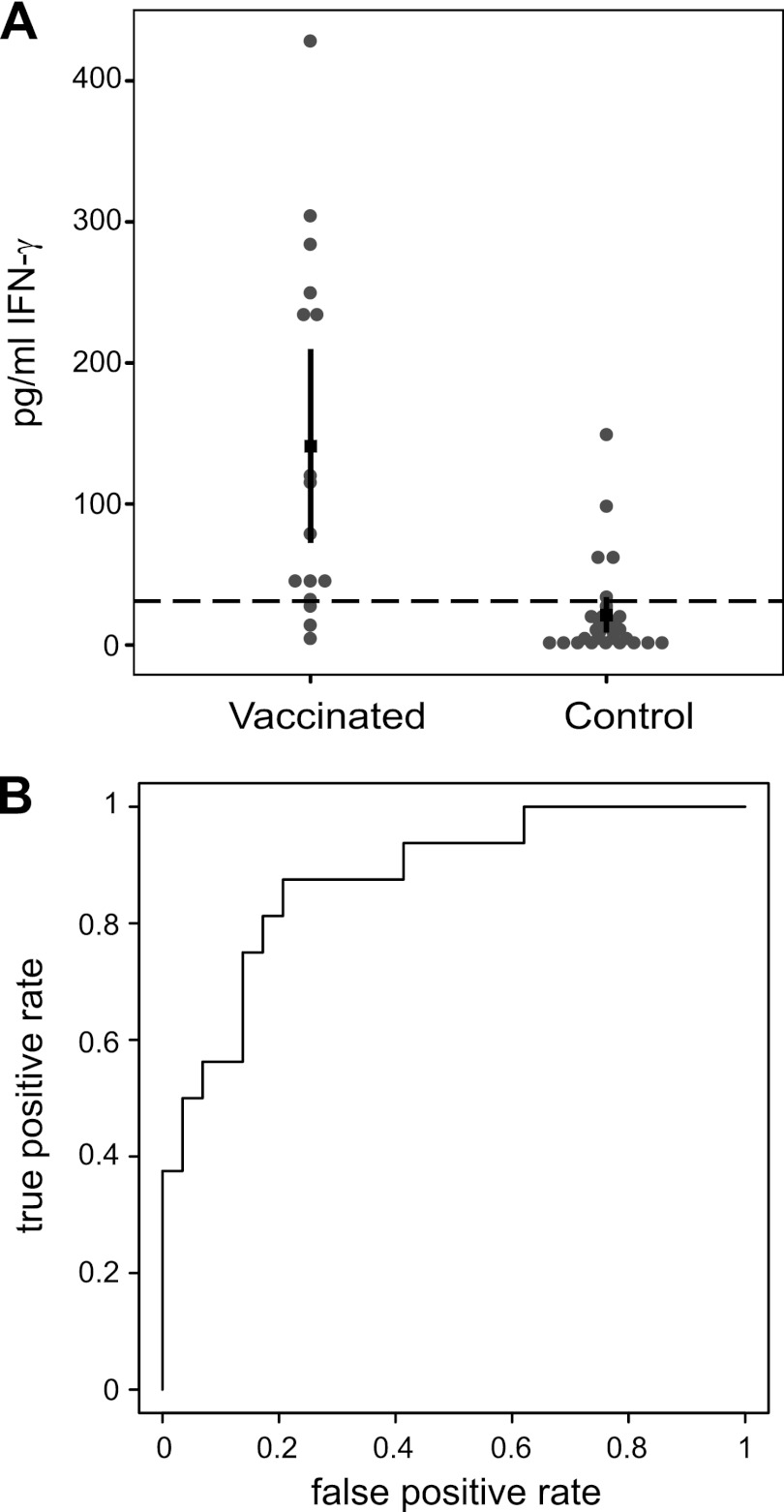
Cellular immunity was evaluated using an IGRA that was developed using a chloroform-methanol residue of phase 1 Nine Mile C. burnetii as an antigen. Whole blood from vaccinated subjects was tested for the ability to produce IFN-γ in response to C. burnetii antigen in vitro. The range of responses in the vaccinated subjects was 4.6 to 428 pg/ml, whereas in the control subjects, the range was 0.24 to 148.91 pg/ml. Individual responses are plotted in Fig. 2A. The mean IFN-γ response in vaccinated subjects was 141.1 pg/ml (95% CI, 72.3 to 210.0 pg/ml), whereas in control subjects, the mean IFN-γ response was 21.4 pg/ml (95% CI, 8.7 to 34.0 pg/ml). The difference of 119.8 pg/ml (95% CI, 50.1 to 189.4 pg/ml) between the two groups was significant. All of the samples tested responded to phytohemagglutinin (PHA) mitogen. To establish a cutoff for a positive response, a ROC curve was constructed (Fig. 2B). Using this curve, a cutoff of 31.11 was chosen to give equal sensitivity and specificity, here resulting in an empirical sensitivity of 81.25% and a specificity of 82.76%. Based on this cutoff, the IGRA therefore found 13/16 (81.25%) vaccinated subjects positive and 5/29 (17.24%) control subjects positive.
Fig 2.
In vitro IFN-γ responses in Q fever-vaccinated and control subjects. IFN-γ production by whole blood in vitro in response to Nine Mile phase 1 CMR antigen was measured. Amounts of IFN-γ produced in the cultures for Q fever-vaccinated and control subjects are shown in panel A. The dashed line represents the cutoff for a positive result. The black square indicates the mean, and the thick vertical lines show the 95% confidence intervals. The cutoff value was determined using a ROC curve analysis. The ROC curve is shown in panel B.
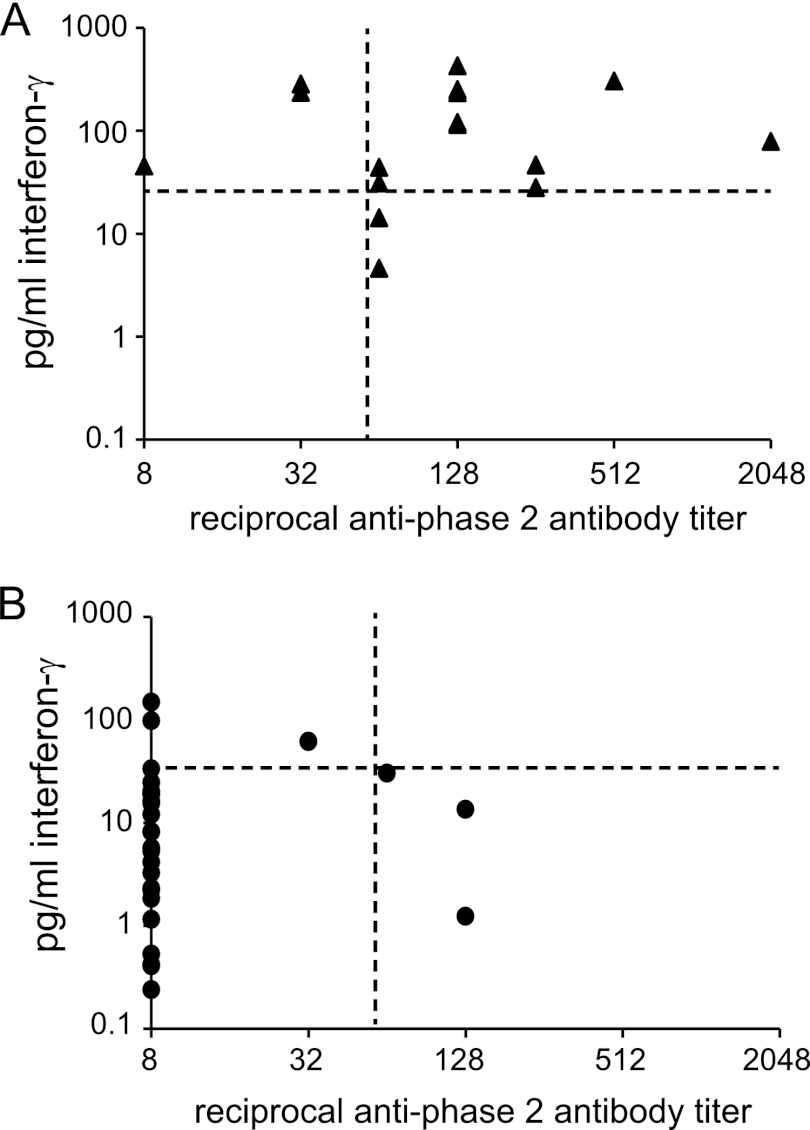
Both the IFA and the IGRA identified 13/16 of the vaccinated subjects as positive, but the 3 subjects that tested negative were different in the different assays. This can be visualized in Fig. 3A, where the vertical and horizontal lines mark the cutoffs for the IFA and IGRA, respectively. There were 3 subjects positive by IGRA only, 3 positive only by the IFA, and 10 samples positive by both assays. None of the vaccinated people were negative by both assays. Figure 3B shows the control group results for both assays. The three subjects that were positive by IFA had negative IGRA responses, and the 5 control subjects that were positive by IGRA were below the cutoff for IFA.
Fig 3.
IFN-γ and IgG responses in Q fever-vaccinated and control subjects. The IFN-γ responses versus the anti-phase 2 IgG titers of vaccinees and controls are shown in panels A and B, respectively. The crossing lines on the graphs indicate the cutoff values for positive results in each assay. Negative results in the IFA were assigned a value of 1:8.
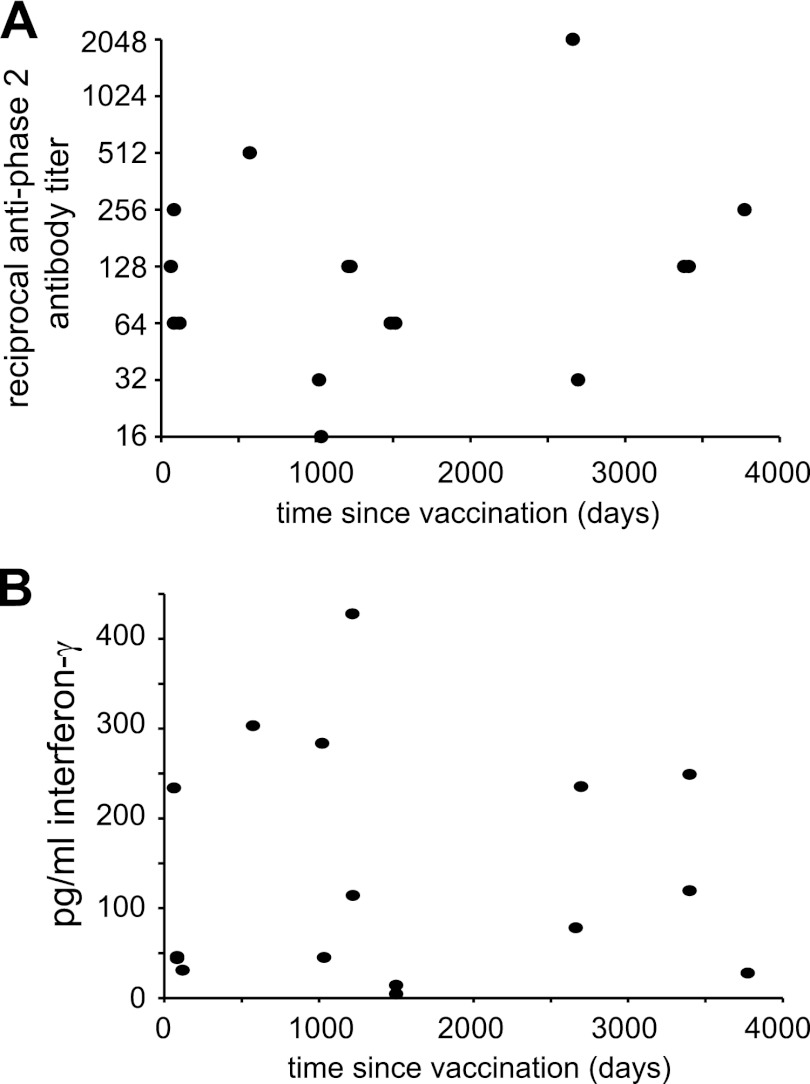
Because the vaccinated subjects were vaccinated over a period of 10 years, it was possible to compare the results of the two assays to the time that had elapsed since vaccination. We plotted each subject's antibody titer against the corresponding number of days since vaccination for that subject (Fig. 4A). Based on this graph, we fit a linear regression model for antibody titer as a function of days since vaccination. The resulting fit for the line did not have a negative slope, indicating that antibody titers were not lower as the time between vaccination and analysis increased. The coefficient for days in this model was estimated as 0.0003 (95% CI, −0.0005 to 0.001; P value = 0.46). Similarly, a plot of IFN-γ production versus days since vaccination (Fig. 4B) did not indicate a statistically significant reduction in IFN-γ responses as the time since vaccination increased. The coefficient for days in this model was estimated as −0.00042 (95% CI, −0.06 to 0.57; P value = 0.98). For these subjects, immune responses to C. burnetii were long-lived, even out to 9 years postvaccination.
Fig 4.
Immune responses in Q fever vaccinees versus time. Plots of the IgG response (A) and the IFN-γ response (B) versus the number of days since vaccination are shown. Fitting of the data in both plots to linear regression models indicated that there was no decline in response over time. The coefficient for days in this model for panel A was estimated as 0.0003 (95% CI, −0.0005 to 0.001; P value = 0.46) and for panel B was −0.00042 (95% CI, −0.06 to 0.57; P value = 0.98).
DISCUSSION
Detection of prior exposure to C. burnetii in humans can be important for the estimation of the impact of Q fever on a population and for defining risk factors for exposure. Exposure to C. burnetii has typically been evaluated by measurement of serum antibody, and this approach has a number of advantages. Serum antibody tests can be performed on a fairly large scale using enzyme-linked immunosorbent assay (ELISA), and the serum can be stored before analysis, allowing archived samples to be analyzed. Measurement of serum antibody is also the “gold standard” for Q fever diagnosis. However, there are some issues with the serum antibody approach. The decline in serum antibody over time could cause seroprevalence to underestimate the true level of exposure in a population significantly, and results of seroprevalence studies can vary significantly depending on the method of antibody detection, the cutoff titer used, and interlaboratory variability (17).
An alternative method that could be employed to examine prior exposure to C. burnetii is the IGRA, which analyzes the T cell response to C. burnetii antigens in vitro. The IGRA has been used for detection of immunity to Mycobacterium tuberculosis, and currently two FDA-approved commercial kits are available for this purpose (18). These assays have been reported to be at least as effective as tuberculin skin tests at detecting exposure to tuberculosis (19). An enzyme-linked immunosorbent spot assay (ELISPOT assay) detecting IFN-γ responses to C. burnetii has also been reported to have positive results on patients with prior acute Q fever and to detect much stronger responses in a small number of chronic Q fever patients (20).
In this study, the abilities of IFA and IGRA to predict prior vaccination against C. burnetii were compared. The two assays performed similarly, with the IFA having a sensitivity of 81.25% and a specificity of 89.66% and the IGRA having a sensitivity of 81.25% and a specificity of 82.76%. Both assays failed to detect a response in 3/16 vaccinated subjects. However, the three nonresponders were different for each assay, therefore resulting in a positive response for all vaccinees when the two assays were used in tandem. Results from the control group showed a positive response in 8/29 subjects when the results of the two assays were combined. Although the use of both assays can bring the sensitivity up to 100%, this can happen only at the expense of specificity, which drops to 72.4% when both assays are used.
There is a possibility that some samples in the control group are true positives and the specificity may not be as poor as it seems. A national seroprevalence study in the United States found 3.1% of people to be seropositive for C. burnetii (21). As this was detected in a broad group of people that did not have any specific risk factors for C. burnetii, it would not be unexpected to find 1 or 2 true positives in the control group used here. However, most of the 8 positives in the control group are likely to be false positives, and the poor specificity when the two assays are used in tandem cannot be overlooked. In this study, the IFA had the best combination of sensitivity and specificity.
These experiments also demonstrate that immune responses to C. burnetii can persist for at least 10 years after vaccination. The subjects vaccinated 7 to 10 years prior to the analysis had responses similar to those of people vaccinated only months before testing. There was not a trend toward lower responses with increased time since vaccination. A caveat to this interpretation is the fact that the subjects vaccinated 7 to 10 years before testing received the IND vaccine developed at the USAMRIID, whereas the more recent vaccinees received Q-Vax from Australia. It is possible that the USAMRIID vaccine induces more robust immune responses and that these have declined over time but are now at the level in the Q-Vax recipients. However, if the responses in Q-Vax recipients are analyzed separately, the data still do not indicate reduced immune responses with increasing time since vaccination.
The results for the IFA that are reported above measure antibodies against the Nine Mile phase 2 strain of C. burnetii. However, IgG antibodies against Nine Mile phase 1 were also detected. For the vaccinated group, 11/16 were positive when 1:64 was used as a cutoff. Levels of anti-phase 1 antibodies are typically very low in acute Q fever infections but can become very high during chronic infections (22). The highest phase 1 titers measured among the vaccinated group were 1:512, below the normal diagnostic criteria for chronic Q fever. For the IGRA, only Nine Mile phase 1 was used to prepare the CMR antigen. It is possible that stronger IGRA responses could have been observed if Nine Mile phase 2 or Henzerling phase 1 (the vaccine strain) was used as the antigen. However, there is no evidence that differences between Nine Mile phase 1 and phase 2 would be apparent in T cell responses to chloroform-methanol-extracted antigens.
Studies on the efficacy of the human Q fever vaccine have suggested that greater than 90% of recipients can be protected (23). However, there is not much information on the longevity of the protection, with the few studies that have been performed estimating protection for at least 5 years (11). It is not currently possible for vaccinated people to receive the vaccine a second time due to adverse reactions at the injection site in previously immune recipients. Studies on people previously infected with C. burnetii have suggested that there may be a decline in immune response over time, at least when antibody titers are measured. It is therefore important to consider how long immune responses will persist in vaccinated people. The results presented here suggest that immune responses can persist for at least 10 years and that measurement of both antibody titers and in vitro T cell responses provides the most sensitive indicator of immunity.
ACKNOWLEDGMENTS
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the CDC or the Department of Health and Human Services.
We thank the blood donors for their contribution to this study.
Footnotes
Published ahead of print 28 November 2012
REFERENCES
- 1. Maurin M, Raoult D. 1999. Q fever. Clin. Microbiol. Rev. 12:518–553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Frankel D, Richet H, Renvoise A, Raoult D. 2011. Q fever in France, 1985–2009. Emerg. Infect. Dis. 17:350–356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. McQuiston JH, Childs JE. 2002. Q fever in humans and animals in the United States. Vector Borne Zoonotic Dis. 2:179–191 [DOI] [PubMed] [Google Scholar]
- 4. Shannon JG, Heinzen RA. 2009. Adaptive immunity to the obligate intracellular pathogen Coxiella burnetii. Immunol. Res. 43:138–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Schneeberger PM, Hermans MH, van Hannen EJ, Schellekens JJ, Leenders AC, Wever PC. 2010. Real-time PCR with serum samples is indispensable for early diagnosis of acute Q fever. Clin. Vaccine Immunol. 17:286–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sukocheva OA, Marmion BP, Storm PA, Lockhart M, Turra M, Graves S. 2010. Long-term persistence after acute Q fever of non-infective Coxiella burnetii cell components, including antigens. Q. J. Med. 103:847–863 [DOI] [PubMed] [Google Scholar]
- 7. Hussain-Yusuf H, Islam A, Healy B, Lockhart M, Nguyen C, Sukocheva O, Stenos J, Graves S. 2012. An analysis of Q fever patients 6 years after an outbreak in Newport, Wales, UK. Q. J. Med. 105:1067–1073 [DOI] [PubMed] [Google Scholar]
- 8. Jerrells TR, Mallavia LP, Hinrichs DJ. 1975. Detection of long-term cellular immunity to Coxiella burneti [sic] as assayed by lymphocyte transformation. Infect. Immun. 11:280–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Read AJ, Erickson S, Harmsen AG. 2010. Role of CD4+ and CD8+ T cells in clearance of primary pulmonary infection with Coxiella burnetii. Infect. Immun. 78:3019–3026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhang G, Russell-Lodrigue KE, Andoh M, Zhang Y, Hendrix LR, Samuel JE. 2007. Mechanisms of vaccine-induced protective immunity against Coxiella burnetii infection in BALB/c mice. J. Immunol. 179:8372–8380 [DOI] [PubMed] [Google Scholar]
- 11. Marmion BP, Ormsbee RA, Kyrkou M, Wright J, Worswick DA, Izzo AA, Esterman A, Feery B, Shapiro RA. 1990. Vaccine prophylaxis of abattoir-associated Q fever: eight years' experience in Australian abattoirs. Epidemiol. Infect. 104:275–287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Committee on Special Immunizations Program for Laboratory Personnel Engaged in Research on Countermeasures for Select Agents 2011. Protecting the frontline in biodefense research: the Special Immunizations Program. The National Academies Press, Washington, DC: [PubMed] [Google Scholar]
- 13. Teunis PF, Schimmer B, Notermans DW, Leenders AC, Wever PC, Kretzschmar ME, Schneeberger PM. 4 April 2012. Time-course of antibody responses against Coxiella burnetii following acute Q fever. Epidemiol. Infect. doi:10.1017/S09502688120004040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Izzo AA, Marmion BP. 1993. Variation in interferon-gamma responses to Coxiella burnetii antigens with lymphocytes from vaccinated or naturally infected subjects. Clin. Exp. Immunol. 94:507–515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Williams JC, Cantrell JL. 1982. Biological and immunological properties of Coxiella burnetii vaccines in C57BL/10ScN endotoxin-nonresponder mice. Infect. Immun. 35:1091–1102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Williams JC, Peacock MG, Waag DM, Kent G, England MJ, Nelson G, Stephenson EH. 1992. Vaccines against coxiellosis and Q fever. Development of a chloroform:methanol residue subunit of phase I Coxiella burnetii for the immunization of animals. Ann. N. Y. Acad. Sci. 653:88–111 [DOI] [PubMed] [Google Scholar]
- 17. Blaauw GJ, Notermans DW, Schimmer B, Meekelenkamp J, Reimerink JH, Teunis P, Schneeberger PM. 2012. The application of an enzyme-linked immunosorbent assay or an immunofluorescent assay test leads to different estimates of seroprevalence of Coxiella burnetii in the population. Epidemiol. Infect. 140:36–41 [DOI] [PubMed] [Google Scholar]
- 18. Mazurek GH, Jereb J, Vernon A, LoBue P, Goldberg S, Castro K. 2010. Updated guidelines for using interferon gamma release assays to detect Mycobacterium tuberculosis infection—United States, 2010. MMWR Recommend. Rep. 59:1–25 [PubMed] [Google Scholar]
- 19. Mazurek GH, LoBue PA, Daley CL, Bernardo J, Lardizabal AA, Bishai WR, Iademarco MF, Rothel JS. 2001. Comparison of a whole-blood interferon gamma assay with tuberculin skin testing for detecting latent Mycobacterium tuberculosis infection. JAMA 286:1740–1747 [DOI] [PubMed] [Google Scholar]
- 20. Limonard GJ, Thijsen SF, Bossink AW, Asscheman A, Bouwman JJ. 2012. Developing a new clinical tool for diagnosing chronic Q fever: the Coxiella ELISPOT. FEMS Immunol. Med. Microbiol. 64:57–60 [DOI] [PubMed] [Google Scholar]
- 21. Anderson AD, Kruszon-Moran D, Loftis AD, McQuillan G, Nicholson WL, Priestley RA, Candee AJ, Patterson NE, Massung RF. 2009. Seroprevalence of Q fever in the United States, 2003–2004. Am. J. Trop. Med. Hyg. 81:691–694 [DOI] [PubMed] [Google Scholar]
- 22. Fournier PE, Thuny F, Richet H, Lepidi H, Casalta JP, Arzouni JP, Maurin M, Celard M, Mainardi JL, Caus T, Collart F, Habib G, Raoult D. 2010. Comprehensive diagnostic strategy for blood culture-negative endocarditis: a prospective study of 819 new cases. Clin. Infect. Dis. 51:131–140 [DOI] [PubMed] [Google Scholar]
- 23. Chiu CK, Durrheim DN. 2007. A review of the efficacy of human Q fever vaccine registered in Australia. N. S. W. Public Health Bull. 18:133–136 [DOI] [PubMed] [Google Scholar]