Abstract
Penicillium marneffei is an emerging human-pathogenic fungus endemic to Southeast Asia. Like a number of other fungal pathogens, P. marneffei exhibits temperature-dependent dimorphic growth and grows in two distinct cellular morphologies, hyphae at 25°C and yeast cells at 37°C. Hyphae can differentiate to produce the infectious agents, asexual spores (conidia), which are inhaled into the host lung, where they are phagocytosed by pulmonary alveolar macrophages. Within macrophages, conidia germinate into unicellular yeast cells, which divide by fission. This minireview focuses on the current understanding of the genes required for the morphogenetic control of conidial germination, hyphal growth, asexual development, and yeast morphogenesis in P. marneffei.
MORPHOGENESIS DURING THE GROWTH AND DEVELOPMENT OF PENICILLIUM MARNEFFEI
Pathogenic fungi have evolved traits that allow them to infect and grow on or in a host. One such trait is the ability to alternate growth forms, where each is suited to a particular environment. This ability is called dimorphism, and it is exhibited by a diverse group of pathogenic fungi (Fig. 1). P. marneffei is an emerging human-pathogenic fungus endemic to Southeast Asia, where it is considered to be AIDS defining. P. marneffei infections occur primarily in individuals with defined immunocompromising conditions; however, a small number of cases of infection in patients without a diagnosed immunodeficiency have also been reported (1, 2). In none of the latter cases has immunocompetency been demonstrated. P. marneffei lacks a defined sexual cycle but possesses all of the genes believed to be required for mating, including both mating type idiomorphs in a heterothallic arrangement among isolates (3). P. marneffei, like a number of other fungal pathogens, exhibits temperature-dependent dimorphic growth, hyphal at 25°C and yeast at 37°C. Exposure to an air interface at 25°C promotes the saprophytic hyphae to differentiate to produce asexual spores (conidia), the infectious agents (Fig. 2). Conidia inhaled into the host lung are phagocytosed by pulmonary alveolar macrophages. Within macrophages, conidia germinate into unicellular yeast cells, which divide by fission (4). This minireview focuses on the current understanding of the genes required for the morphogenetic control of conidial germination, hyphal growth, asexual development, and yeast morphogenesis in P. marneffei (Table 1). It should be noted that P. marneffei has recently been renamed Talaromyces marneffei on the basis of new molecular phylogenetic analyses (5).
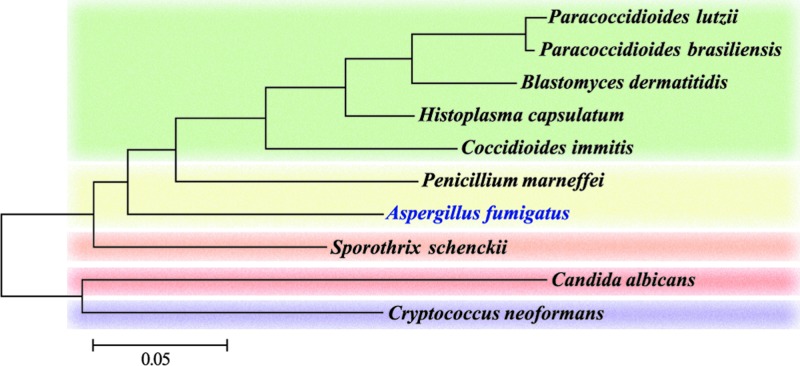
Fig 1.
Evolutionary relationships of dimorphic human pathogens. The evolutionary tree shown is based on the conserved glycerol 3-phosphate dehydrogenase (GpdA) protein from all of the major dimorphic human pathogens and the monomorphic pathogen A. fumigatus for reference. The tree was inferred by using the maximum-likelihood method based on the JTT matrix-based model, and the scale shows the number of amino acid substitutions per site (50). With the exception of Cryptococcus neoformans, which is in the phylum Basidiomycota, all of the other species are in the phylum Ascomycota. These species cover a diverse range of orders (Onygenales, green; Eurotiales, yellow; Ophiostomales, orange; and Saccharomycetales, red).
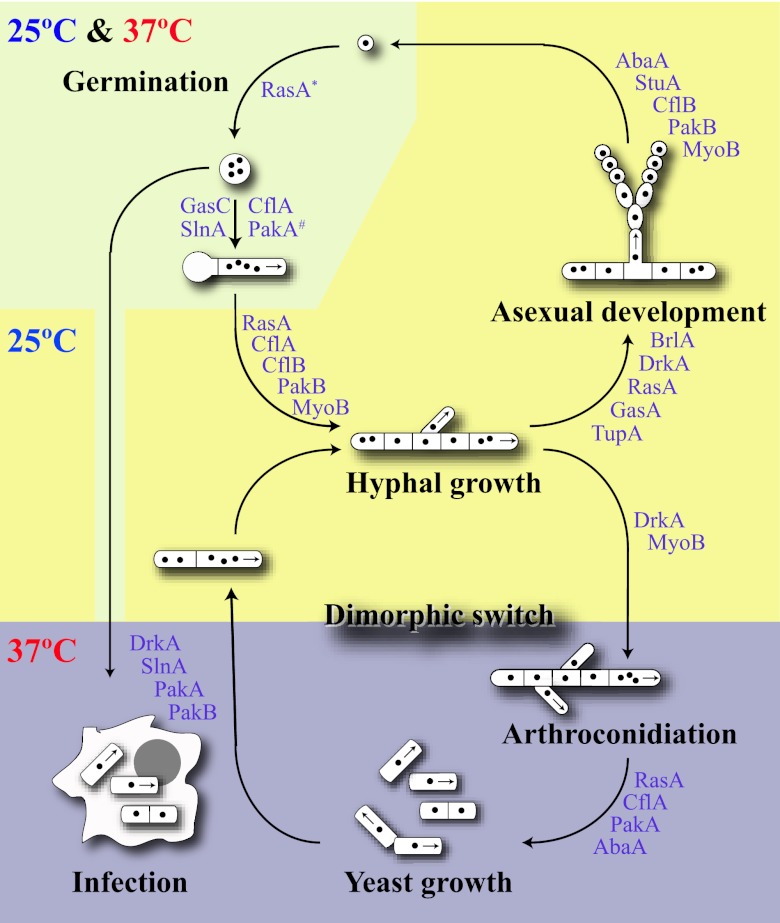
Fig 2.
Life cycle of P. marneffei. Shown is a representation of the life cycle of P. marneffei showing the processes of germination that occur similarly at 25 and 37°C, vegetative hyphal growth and asexual development that occur at 25°C, and arthroconidiation and vegetative yeast growth that occur at 37°C. The genes involved in the various morphogenetic and developmental steps depicted are indicated where appropriate and are described in the text. The # symbol denotes activity only at 25°C.
Table 1.
Genes required for the growth and morphogenesis of P. marneffei
| Gene | Product | Role(s) | Reference |
|---|---|---|---|
| gasA | Gα subunit | Negative regulation of asexual development, positive regulation of secondary metabolism | 22 |
| gasC | Gα subunit | Negative regulation of asexual development, positive regulation of secondary metabolism, germination | 9 |
| rasA | Ras GTPase | Negative regulation of onset of asexual development, conidial germination, polarized growth of hyphae and yeast cells | 8 |
| cflA | Rho GTPase | Conidial germination, polarized growth of hyphae and yeast cells, actin concentrated at cell apices | 7 |
| cflB | Rho GTPase | Polarized growth of hyphae and conidiophores, actin concentrated at cell apices, germination in vitro | 31 |
| pakA | PAK | Germination, yeast morphogenesis | 6 |
| pakB | PAK | Promotion of hyphal growth, formation of septa at phialide-to-conidium boundary in conidiophores, yeast morphogenesis during macrophage infection (but not in vitro yeast morphogenesis) | 32 |
| myoB | Type II myosin | Cytokinesis and chitin deposition at septa; hyphal, conidiophore, and yeast morphogenesis | 45 |
| tupA | Tup1p/GROUCHO-related WD40 transcription factor | Repression of secondary metabolism, asexual development, yeast growth and morphogenesis | 40 |
| tpbA | TATA binding transcription factor | Essential for hyphal growth | 51 |
| drkA | HHK (class III) | Hyphal morphogenesis; response to osmotic, oxidative, and cell wall stress; resistance to antifungal agents; promotion of asexual development; production of yeast cells (dimorphic switching) | 24 |
| slnA | HHK (class VI) | Germination; hyphal morphogenesis; response to osmotic, oxidative and cell wall stress; promotion of asexual development | 24 |
| hhk1 | HHK (class X) | Germination, promotion of asexual development, cell wall integrity | 25 |
| abaA | ATTS transcription factor | Formation of conidia during asexual development | 38 |
| stuA | APSES transcription factor | Spatial organization of developing conidiophore (production of metulae and phialides) | 41 |
| sltA | C2H2 zinc finger transcription factor | None determined but can functionally complement Aspergillus nidulans steA mutant | 52 |
| rfxA | RFX transcriptional regulator | Essential, required for growth and nuclear division | 44 |
| areA | GATA transcription factor | Growth on nonpreferred nitrogen sources, production of secreted proteases | 48 |
| acuD | Isocitrate lyase | Acetate and fatty acid utilization | 53 |
CONSERVED SIGNALING AND CELL POLARITY FACTORS ARE REQUIRED FOR CONIDIAL GERMINATION IN P. MARNEFFEI
Under favorable environmental conditions, dormant conidia (asexual spores) germinate at 25°C to give rise to the extensive radiating hyphal network necessary for saprophytic colonization or at 37°C to produce pathogenic yeast cells capable of causing disease in a human host. Germination requires an initial period of isotropic growth, followed by the establishment of a polarized axis to allow germ tube emergence. In P. marneffei, germination is regulated by the heterotrimeric G-protein α subunit GasC, the Ras and Rho GTPases RasA and CflA, and the downstream p21-activated kinase (PAK) PakA (6–9) (Table 1; Fig. 2). The gasC orthologues, rasA, and cflA are also important for conidial germination in the taxonomically closest model fungus Aspergillus nidulans, in Botrytis cinerea, and in a variety of other fungal species (3, 10–21).
Canonical heterotrimeric G proteins are composed of three subunits (α, β, and γ) which interact with receptors to transmit environmental signals. GDP-to-GTP exchange in the Gα subunit is triggered by ligand binding to the associated receptor and results in a conformational change and dissociation from the β and γ subunits. The P. marneffei genome contains three Gα subunit-encoding genes (gasA, gasB, and gasC), one Gβ subunit-encoding gene (sfaD), and one Gγ subunit-encoding gene (gpgA). gasC, but not gasA or gasB, is required for conidial germination at both 25 and 37°C (6, 9, 22) (S. Zuber and A. Andrianopoulos, unpublished data). At both 25 and 37°C, ΔgasC and gasCG207R dominant negative mutants show slower germination rates than the wild type, while the gasCG45R dominant activated mutant has accelerated germination (9). GasC acts upstream of the RAS GTPase RasA, the Cdc42p orthologue CflA, and the downstream PAK PakA (Table 1). However, the roles of these downstream signaling components differ at 25 and 37°C. Both dominant negative rasAD125A and dominant activated rasAG19V mutants show delayed germination at 25°C, producing large, swollen conidia, but germination at 37°C is unaffected (6, 8), whereas cflAD120A dominant negative and cflAG14V dominant activated mutants show decreased and increased germination rates, respectively, at both 25 and 37°C (6, 7). The cflAG14V allele can suppress the germination defects in a rasAD125A mutant at 25°C, suggesting that CflA acts downstream of RasA at 25°C but not at 37°C (8).
Deletion of pakA or mutation of the Cdc42-Rac-interacting binding domain (CRIB) required for interaction with Rho GTPases, pakAH108G, results in only a minor decrease in germination at 25°C but an almost complete absence of germination at 37°C (6). Both ΔpakA and pakAH108G allele mutants suppress the accelerated germination of the cflAG14V mutant at 25 and 37°C, suggesting that PakA acts downstream of CflA (6). The differing roles of RasA, CflA, and PakA at 25 and 37°C during hyphal and yeast morphogenesis, respectively, suggest that the circuitry and downstream effectors either differ or are differentially regulated. One clue as to the basis for this fundamental temperature-dependent regulation comes from the observation that the germination defect in the ΔpakA mutant at 37°C gradually decreases with temperature, as opposed to a sharp change at a threshold temperature (6). This points to control by thermodynamic stability of complexes, as opposed to the tightly regulated expression of a temperature-specific factor. Moreover, signals mediated by GasC trigger both RasA-CflA-PakA-dependent and -independent pathways to control germination, as the ΔpakA mutation only partially suppresses the accelerated germination of the gasCG45R mutant (6). This hypothesis is supported by the germination rates of a ΔpakA gasCG207R mutant, which are lower than those of both of the single mutant strains, suggesting an additive effect (6). In A. nidulans, cyclic AMP (cAMP) and RAS pathways independently regulate germination (23). While it is likely that the second pathway in P. marneffei involves cAMP signaling, cAMP supplementation or addition of the phosphodiesterase inhibitor theophylline cannot suppress the ΔgasC mutant's delayed-germination phenotype (9).
Recently, novel roles for sensor hybrid histidine kinases (HHKs) during conidial germination in P. marneffei have been described (24, 25) (Table 1). Histidine kinases are alternative sensors to G-protein-coupled receptors for signaling changes in the external environment and often act via the activation of mitogen-activated protein kinase (MAPK) pathways. Reduced expression, because of RNA interference, of the class X HHK encoded by hhkJ (hhk1) reduces germination at both 25 and 37°C (25). Deletion of the class VI HHK encoded by slnA also results in a dramatic decrease in germination at both 25 and 37°C (24). In Saccharomyces cerevisiae, the slnA orthologue SLN1, in combination with the Sho1p osmosensor, leads to the activation of Ste11p (MAPK kinase kinase) in a process that involves Cdc42p (CflA orthologue) and Ste20p (PakA orthologue) (26, 27). HhkJ or SlnA may interact with CflA and/or PakA to activate the P. marneffei Ste11p orthologue SteC. Deletion of P. marneffei slnA has been shown to affect the phosphorylation levels of MAPK SakA (Hog1 orthologue), which has been proposed to act downstream of SteC (24, 28). SakA phosphorylation levels have recently been shown to regulate the transition between spore dormancy and germination in A. nidulans (29).
MORPHOGENETIC CONTROL OF HYPHAL GROWTH AND POLARITY
Once a conidium has extended a primary germ tube, polarized growth continues along the pre-established axis to produce the highly polarized hyphal cell. Hyphae undergo cytokinesis without cellular division to become septate and branch at division nodes. Subapical cells are predominately uninucleate, whereas apical cells are multinucleate. Polarized hyphal growth requires polarized actin at the cellular apex. Rho GTPases, orthologous to Cdc42 and Rac, play conserved roles in the regulation of actin-mediated polarized growth in fungi. However, the relative role of each GTPase in either hyphal growth or asexual development varies dramatically between species (reviewed in reference 30). In P. marneffei, both CflA (Cdc42) and CflB (Rac) are required for polarized growth of hyphae, whereas only CflB is required for polarized growth of conidiophores (7, 31) (Table 1; Fig. 2). Dominant negative cflAD120A and activated cflAG14V mutants produce misshapen, swollen, and multinucleate hyphal cells displaying increased septa (7). Deletion of the related Rho GTPase (Rac) gene cflB also results in aberrantly shaped, swollen, highly septate, multinucleate hyphal cells that are additionally hyperbranched (31). Mutation of both cflA and cflB results in no actin concentrated at the hyphal apex (7, 31). The phenotype caused by the dominant activated cflAG14V allele can be suppressed by a dominant negative rasAD125A allele, suggesting that CflA acts downstream of RasA during polarized hyphal growth (8). The dominant activated rasAG19V or cflAG14V alleles can suppress the hyperbranching but not the polarity phenotype of the ΔcflB mutant, indicating that CflA and CflB play unique roles (8).
Rho GTPases establish polarized growth not only by recruiting actin but by activating PAKs to regulate cellular division and proteins required for polarized growth, in addition to signaling cascades that culminate in changes in gene expression. Recent studies have shown that pakA (Ste20p) plays a role during polarized conidial germination (discussed above), whereas pakB (Cla4p) is required for the signal to grow in the hyphal form at 25°C (6, 32). Interestingly, deletion of pakB results in inappropriate yeast growth at 25°C, with colonies appearing yeastlike (compact and mucoid), with individual yeast cells evident (32). While the ΔpakB mutant shows decreased expression of hypha-specific genes and increased expression of yeast-specific genes at 25°C, deletion of brlA (primary regulator of asexual development) from the ΔpakB mutant strain prevents the formation of yeast cells, suggesting that these cells arise inappropriately from the conidiation program (32). Deletion of the asexual development transcriptional repressor tupA also results in inappropriate production of yeast cells at 25°C (33). However, the decreased expression of hyphal genes in the ΔpakB mutant suggests that PakB is actively signaling to promote hyphal growth, whereas TupA is repressing the expression of genes required for yeastlike growth and also indicates that yeast-type morphology is the default growth state. Expression of pakB mutant alleles with deletion of the CRIB domain (required for interaction with Rho GTPases) or the Gβ binding (GBB) domain (required for interaction with the β subunit of heterotrimeric G proteins) could not suppress the hyperbranched, tightly packed hyphal phenotype of the ΔpakB mutant, suggesting that interaction of PakB with CflA or CflB and SfaD is essential for hyphal morphogenesis (32). Unlike PakA, which colocalizes with actin at discrete sites, PakB is localized as a cap at the hyphal apex, suggesting that it may also activate the polarisome (6, 32).
REGULATION OF THE ONSET AND MORPHOGENESIS OF ASEXUAL DEVELOPMENT
Exposure of hyphal cells to an air interface and light at 25°C promotes asexual differentiation to produce asexual spores (conidia). While the molecular mechanism by which these inducing signals trigger development in P. marneffei remains to be uncovered, the heterotrimeric Gα subunits gasA and gasC (to a lesser extent) and downstream RasA are known to regulate the onset of asexual development (8, 9, 22) (Fig. 2). Mutants carrying the gasAG42R or gasCG45R dominant activated allele display reduced conidiation, whereas those with the dominant negative gasAG203R and ΔgasC alleles show increased conidiation (9, 22). The dominant negative rasAD125A allele results in the early onset of conidiation (8). GasA, GasC, and RasA are thought to activate a PKA signaling pathway that negatively regulates the expression of the primary regulator of asexual development brlA and its downstream target regulator abaA (22). Sensor HHKs also regulate the onset and extent of asexual development in P. marneffei via the brlA regulatory pathway. Deletion of drkA and, to a lesser extent, slnA results in a delay in the onset of conidiation and a decrease in conidial density (24). Consistent with this, the ΔdrkA mutant showed dramatically reduced levels of brlA expression under conditions expected to induce asexual development. Reduced expression of a third HHK in P. marneffei, hkk1, also reduces conidiation levels, suggesting significant functional overlap between these kinases (25). The DRK1 orthologues in Blastomyces dermatitidis, B. cinerea, and A. nidulans also regulate the extent of asexual development (34–36).
Conidiophores are produced from a specialized stalk from which differentiated uninucleate cells are produced sequentially in a budding fashion: metulae bud from the stalk, phialides bud from metulae, and uninucleate conidia bud from phialides. This developmental program is homologous to the well-characterized A. nidulans conidiation program and is regulated in a similar manner, despite the morphological differences between the conidiophores of the two genera (37, 38). The molecular components of the central regulatory pathway controlling differentiation are also conserved. Deletion of brlA and abaA in P. marneffei prevents the formation of conidia (38). The ΔbrlA mutant produces only conidiophore stalks, whereas the ΔabaA mutant fails to undergo the shift from acropetal to basipetal division, resulting in a lack of phialides and conidia (A. R. Borneman and A. Andrianopoulos, unpublished data) (38). In contrast, the TupA transcriptional corepressor in P. marneffei is required to repress the brlA regulatory pathway and deletion of tupA results in premature conidiation and expression of brlA, while the opposite is true for the A. nidulans orthologue rcoA (39, 40). Expression of drkA is increased in the ΔbrlA mutant and reduced in the ΔabaA mutant, showing that feedback regulatory loops exist that control the signaling pathways, in addition to the loops between brlA and abaA that are known to operate in A. nidulans. Interestingly, while brlA is conserved throughout the class Ascomycetes, the abaA gene appears to have been lost in the order Onygenales, which encompasses many of the dimorphic pathogens, such as Histoplasma capsulatum.
A number of genes that affect overall conidiophore morphology or cell type morphogenesis in P. marneffei have been characterized. Deletion of the developmental modifier stuA results in conidiophores that lack metulae and phialides. In the ΔstuA mutant, conidia bud directly off the stalk, indicating that StuA is required for the spatial organization of the developing conidiophore, as has been shown for A. nidulans (41–43). The aberrant placement of nuclei also has an impact on conidiophore differentiation. Reduced expression of the nuclear division transcriptional regulator rfxA results in multinucleate conidiophores containing aberrant nuclei that lack differentiated sterigmata (44). The Rho GTPase CflB, as well as being required for polarized growth of hyphae, is required for polarized growth of the conidiophore structure. Conidiophores of the ΔcflB mutant are difficult to distinguish from the aberrant hyphae, as metulae and phialides are swollen, misshapen, and multinucleate (31). CflB is required for polarized actin at the cell apices of conidiophore cell types (31). More recently, genes that are required for the formation of the septa that separate the conidiophore cell types have been characterized. Deletion of the type II myosin gene myoB results in conidiophores that lack clearly defined cell types. Typically, stalks produced a “phialide-like” cell from which a single terminal conidium was observed and conidiophores lacked or possessed malformed septa separating the metula-phialide and conidium-phialide boundaries (45). This suggests that the sequential production of metulae, phialides, and conidia by budding is contingent on the successful completion of septation. ΔmyoB conidiophores also contained nuclei with aberrant morphology, suggesting that the conidiophore septa may be playing a spatial role in the placement of nuclei in the conidiophore (45). Deletion of the PAK encoded by pakB results in conidiophores with normal metulae and phialides but only a single abnormally large terminal conidium (32). In contrast to the wild type, in which two chitin discs can be observed at the phialide-to-conidium boundary, only one, no, or incomplete septa are seen at the phialide-to-conidium boundary in ΔpakB mutant conidiophores. This suggests that PakB is required for septation and cell separation of conidia from phialides. ΔpakB conidia also show abnormal chitin distribution (32). The role of pakB in septation and cell separation of conidia from phialides is conserved in the Magnaporthe grisea orthologue CHM1 (46).
IN VITRO AND IN VIVO YEAST MORPHOGENESIS
When switched to 37°C in vitro, P. marneffei undergoes a morphogenetic transition termed arthroconidiation (Fig. 2). During arthroconidiation, cellular division and nuclear division become coupled, double septa are laid down, and hyphae fragment at septation sites to liberate uninucleate yeast cells, which subsequently divide by fission. During infection, the arthroconidiation process is bypassed. Conidia inhaled by the host are phagocytosed by pulmonary alveolar macrophages and germinate directly into the unicellular yeast cells in these host cells (4, 24). Both pH and nitrogen influence the transition to yeast in vitro but are not sufficient to trigger the process. Hyphal growth at 28°C is optimal at a pH of 5 to 7, whereas a pH of 4 to 6 is optimal for yeast growth at 37°C. Compared to both neutral and alkaline pHs, an acidic pH increases the rate of hypha-to-yeast conversion (47). The nitrogen source also influences the transition to yeast at 37°C. The utilization of nonpreferred nitrogen sources has been shown to enhance filamentation at 37°C; however, this enhancement appears to be independent of the global regulator areA, which encodes the GATA transcription factor required for the utilization of nonpreferred nitrogen sources. Deletion of areA does not decrease filamentation or affect yeast cell production (40, 48).
Sensing of either the temperature shift to 37°C or the presence of the host intracellular environment is required for induction of the morphological transition of P. marneffei to yeast growth. Both sensor HHKs DrkA and SlnA are required for yeast growth at 37°C (24). The ΔslnA mutant has delayed conidial germination at 37°C, and both the ΔslnA and ΔdrkA mutants produce aberrant arthroconidiating hyphal cells that are swollen and display fewer septa in vitro (24). Only a very small number of aberrantly shaped and swollen yeast cells are produced (24). Concomitant with the in vitro phenotype, both the ΔslnA and ΔdrkA mutants are unable to produce yeast cells in macrophages after 24 h, with phagocytosed conidia remaining ungerminated or predominately converting to germlings, respectively (24). The role of DrkA in the regulation of the transition to yeast growth is conserved in B. dermatitidis and H. capsulatum, dimorphic pathogens with a budding mode of division that also lack a class VI Sln1p orthologue (34). These results suggest that DrkA and SlnA are involved in sensing at 37°C and signaling to a downstream pathway to regulate the morphological transition to yeast growth, but it is not yet clear to which aspect of this signal they are responding. This pathway is likely to include the downstream response regulators orthologous to S. cerevisiae Skn7p and Ssk1p and one or more MAPK pathways. In S. cerevisiae, in response to osmotic stress, the single sensor HHK Sln1p (SlnA orthologue) phosphorylates the response regulators Ssk1p and Skn7p via the Ypd1 phosphotransfer protein. Ssk1p dephosphorylation triggers the activation of the Ssk2p/Ssk22p-Pbs2p-Hog1p MAPK pathway, whereas Skn7p binds directly to DNA to regulate transcription (reviewed in reference 27). Deletion of DrkA in P. marneffei has been shown to be essential for the increase in the phosphorylation of the SakA (Hog1p orthologue) and MpkA (Slt2p orthologue) MAPKs, most likely via the action of the Ssk1p orthologue SskA (24). The P. marneffei Skn7p orthologue SrrA is also likely to have a conserved role in transcriptional regulation, as it can complement the oxidative-stress sensitivity exhibited by the S. cerevisiae skn7 disruptant strain (49).
A tight coupling of cellular division and nuclear division is essential for arthroconidiation and the production of yeast cells at 37°C. Therefore, mutations in genes involved in this process leads to a loss of yeast cell production. Deletion of the type II myosin gene essential for cytokinesis, myoB, leads to a loss of arthroconidiation, and the deletion mutant therefore continues to grow as aseptate, branched, and aberrant hyphae at 37°C (45). Haploinsufficiency or depletion of rfxA, which encodes the RFX transcription factor that regulates nuclear division, blocks yeast cell morphogenesis, and only swollen, irregularly shaped arthroconidial hyphae with nuclear or cell lysis defects are produced. Overexpression of rfxA results in a complete lack of growth at 37°C (44).
In addition to regulating actin-mediated polarized growth of hyphae, the Ras and Rho GTPases also control yeast morphogenesis. Both dominant active and negative alleles for rasA or cflA lead to the production of aberrant yeast cells with shape, size, branching, septation, and chitin deposition defects (7, 8). The constitutively active cflAG14V allele suppresses the effect of the inactive rasAD125A allele, placing CflA downstream of RasA in the pathway regulating yeast cell morphogenesis (8). Interestingly, although cflA is required for in vitro yeast morphogenesis, cflAD120A and cflAG14V mutants produce numerous yeast cells with wild-type morphology during macrophage infection, showing that the in vitro defects are most likely a result of the defects in arthroconidiation rather than subsequent yeast growth (32). The converse is true for the related Rac GTPase CflB. The ΔcflB mutant produces yeast cells with wild-type morphology in vitro at 37°C, but conidia fail to germinate in a macrophage infection assay (31, 32). The PAKs that act downstream of Rho GTPases, encoded by pakA (Ste20p) and pakB (Cla4p), are also required for yeast morphogenesis. Deletion of pakA decreases conidial germination in vitro and during macrophage infection, and the arthroconidiating hyphae and yeast cells that are eventually produced in vitro are swollen, with increased septation (6). Expression of the pakAH108G allele (CRIB domain mutation), which is predicted to prevent CflA-PakA interaction, also results in conidial germination defects at 37°C and swollen arthroconidiating hyphae and yeast cells in vitro, suggesting that PakA is acting downstream of CflA during these processes (6). Interestingly, like the deletion of cflB, that of pakB does not affect in vitro yeast morphogenesis but has dramatic effects during in vivo yeast growth in macrophages. Macrophages infected with ΔpakB conidia contain predominantly highly branched hyphal cells and no yeast cells, indicating that PakB is required for yeast cell division during infection (32). PakB appears to be required for cell separation rather than cytokinesis, as hyphal cells are septate and PakB is specifically localized to septa and adjacent to the division site only after cell wall deposition has occurred (32). The mechanism by which PakB regulates yeast cell morphogenesis inside the host remains unclear. The ΔpakB pakBΔCRIB and ΔpakB pakBΔGBB mutants produce both septate yeast and hyphal cells during infection, suggesting that the interaction with Rho GTPases and heterotrimeric G proteins plays a partial but not essential role in PakB regulation in vivo (32). Interaction is likely to be with CflB rather than CflA, as cflA mutants have no phenotype during macrophage infection (32). However, the ΔcflB mutant fails to germinate in macrophages and the pakBH204G or pakBΔCRIB mutation does not recapitulate this phenotype or that of the ΔpakB mutant, suggesting that PakB is also regulated by Rho-independent mechanisms (32). This pathway seems to be responding to host-derived inductive signals rather than temperature and provides an exciting avenue for further investigation.
CONCLUSIONS
Many genes that are required for P. marneffei growth, morphogenesis, and development have been identified and characterized. Their molecular and cellular roles have been uncovered, as have their interrelationships. A number of these genes are highly conserved in other dimorphic fungi or other related monomorphic fungi. While their molecular function is, more often than not, highly conserved, their cellular roles in a number of instances have diverged. For example, conserved signaling and cell polarity factors play important roles during the various developmental and growth stages of P. marneffei but there are clear differences in how the downstream regulatory pathways operate. Also, conidial germination requires both HHK and heterotrimeric G-protein–Ras signaling, as well as the establishment of actin-mediated polarized growth through the action of a Rho GTPase, but the cellular phenotypes of mutants are often very different. This is not surprising, given the nature of natural selection and evolution. The sample size, in terms of similar studies of the same genes in different fungi, is unfortunately too small to allow strong conclusions about selective pressures.
For the dimorphic fungi, and P. marneffei in particular, these studies have highlighted the complex interplay between asexual development and yeast morphogenesis, showing that the overlapping mechanisms are not just restricted to in vitro hypha-to-yeast dimorphic switching but also extend to morphogenesis inside host cells (24, 38). This is despite the differences in yeast cell morphogenesis in vitro and in host cells in P. marneffei. Many questions remain, such as the exact nature of the signals that trigger the dimorphic switch in response to temperature and the host cell and how these various gene products are integrated into signaling and response pathways to effect the transition. A particularly exciting avenue of research into the issue of host cell signals has been opened by studies of the PakB-dependent pathway that is required for yeast morphogenesis during macrophage infection, appears to respond to host signals rather than temperature, and is only partially dependent on conserved Rho GTPases. Exploration of the molecular nature of this pathway during the morphogenesis of P. marneffei pathogenic yeast cells and how it operates in other dimorphic pathogens will be of great interest.
Footnotes
Published ahead of print 30 November 2012
REFERENCES
- 1. Chan YF, Chow TC. 1990. Ultrastructural observations on Penicillium marneffei in natural human infection. Ultrastruct. Pathol. 14:439–452 [DOI] [PubMed] [Google Scholar]
- 2. Cao L, Chen DL, Lee C, Chan CM, Chan KM, Vanittanakom N, Tsang DN, Yuen KY. 1998. Detection of specific antibodies to an antigenic mannoprotein for diagnosis of Penicillium marneffei penicilliosis. J. Clin. Microbiol. 36:3028–3031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dyer PS, O'Gorman CM. 2011. A fungal sexual revolution: Aspergillus and Penicillium show the way. Curr. Opin. Microbiol. 14:649–654 [DOI] [PubMed] [Google Scholar]
- 4. Vanittanakom N, Cooper CR, Jr, Fisher MC, Sirisanthana T. 2006. Penicillium marneffei infection and recent advances in the epidemiology and molecular biology aspects. Clin. Microbiol. Rev. 19:95–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Samson RA, Yilmaz N, Houbraken J, Spierenburg H, Seifert KA, Peterson SW, Varga J, Frisvad JC. 2011. Phylogeny and nomenclature of the genus Talaromyces and taxa accommodated in Penicillium subgenus Biverticillium. Stud. Mycol. 70:159–183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Boyce KJ, Andrianopoulos A. 2007. A p21-activated kinase is required for conidial germination in Penicillium marneffei. PLoS Pathog. 3:e162 doi:10.1371/journal.ppat.0030162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Boyce KJ, Hynes MJ, Andrianopoulos A. 2001. The CDC42 homolog of the dimorphic fungus Penicillium marneffei is required for correct cell polarization during growth but not development. J. Bacteriol. 183:3447–3457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Boyce KJ, Hynes MJ, Andrianopoulos A. 2005. The Ras and Rho GTPases genetically interact to co-ordinately regulate cell polarity during development in Penicillium marneffei. Mol. Microbiol. 55:1487–1501 [DOI] [PubMed] [Google Scholar]
- 9. Zuber S, Hynes MJ, Andrianopoulos A. 2003. The G-protein α-subunit GasC plays a major role in germination in the dimorphic fungus Penicillium marneffei. Genetics 164:487–499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bluhm BH, Zhao X, Flaherty JE, Xu JR, Dunkle LD. 2007. RAS2 regulates growth and pathogenesis in Fusarium graminearum. Mol. Plant Microbe Interact. 20:627–636 [DOI] [PubMed] [Google Scholar]
- 11. Chang MH, Chae KS, Han DM, Jahng KY. 2004. The GanB Galpha-protein negatively regulates asexual sporulation and plays a positive role in conidial germination in Aspergillus nidulans. Genetics 167:1305–1315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chen C, Ha YS, Min JY, Memmott SD, Dickman MB. 2006. Cdc42 is required for proper growth and development in the fungal pathogen Colletotrichum trifolii. Eukaryot. Cell 5:155–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Doehlemann G, Berndt P, Hahn M. 2006. Different signalling pathways involving a Galpha protein, cAMP and a MAP kinase control germination of Botrytis cinerea conidia. Mol. Microbiol. 59:821–835 [DOI] [PubMed] [Google Scholar]
- 14. Fortwendel JR, Fuller KK, Stephens TJ, Bacon WC, Askew DS, Rhodes JC. 2008. Aspergillus fumigatus RasA regulates asexual development and cell wall integrity. Eukaryot. Cell 7:1530–1539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. García-Rico RO, Martin JF, Fierro F. 2011. Heterotrimeric Galpha protein Pga1 from Penicillium chrysogenum triggers germination in response to carbon sources and affects negatively resistance to different stress conditions. Fungal Genet. Biol. 48:641–649 [DOI] [PubMed] [Google Scholar]
- 16. Kokkelink L, Minz A, Al-Masri M, Giesbert S, Barakat R, Sharon A, Tudzynski P. 2011. The small GTPase BcCdc42 affects nuclear division, germination and virulence of the gray mold fungus Botrytis cinerea. Fungal Genet. Biol. 48:1012–1019 [DOI] [PubMed] [Google Scholar]
- 17. Lafon A, Seo JA, Han KH, Yu JH, d'Enfert C. 2005. The heterotrimeric G-protein GanB(alpha)-SfaD(beta)-GpgA(gamma) is a carbon source sensor involved in early cAMP-dependent germination in Aspergillus nidulans. Genetics 171:71–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Schumacher J, Kokkelink L, Huesmann C, Jimenez-Teja D, Collado IG, Barakat R, Tudzynski P, Tudzynski B. 2008. The cAMP-dependent signaling pathway and its role in conidial germination, growth, and virulence of the gray mold Botrytis cinerea. Mol. Plant Microbe Interact. 21:1443–1459 [DOI] [PubMed] [Google Scholar]
- 19. Som T, Kolaparthi VS. 1994. Developmental decisions in Aspergillus nidulans are modulated by Ras activity. Mol. Cell. Biol. 14:5333–5348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Virag A, Lee MP, Si H, Harris SD. 2007. Regulation of hyphal morphogenesis by Cdc42 and Rac1 homologues in Aspergillus nidulans. Mol. Microbiol. 66:1579–1596 [DOI] [PubMed] [Google Scholar]
- 21. Zheng W, Zhao Z, Chen J, Liu W, Ke H, Zhou J, Lu G, Darvill AG, Albersheim P, Wu S, Wang Z. 2009. A Cdc42 ortholog is required for penetration and virulence of Magnaporthe grisea. Fungal Genet. Biol. 46:450–460 [DOI] [PubMed] [Google Scholar]
- 22. Zuber S, Hynes MJ, Andrianopoulos A. 2002. G-protein signaling mediates asexual development at 25°C but has no effect on yeast-like growth at 37°C in the dimorphic fungus Penicillium marneffei. Eukaryot. Cell 1:440–447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fillinger S, Chaveroche MK, Shimizu K, Keller N, d'Enfert C. 2002. cAMP and ras signalling independently control spore germination in the filamentous fungus Aspergillus nidulans. Mol. Microbiol. 44:1001–1016 [DOI] [PubMed] [Google Scholar]
- 24. Boyce KJ, Schreider L, Kirszenblat L, Andrianopoulos A. 2011. The two-component histidine kinases DrkA and SlnA are required for in vivo growth in the human pathogen Penicillium marneffei. Mol. Microbiol. 82:1164–1184 [DOI] [PubMed] [Google Scholar]
- 25. Wang F, Tao J, Qian Z, You S, Dong H, Shen H, Chen X, Tang S, Ren S. 2009. A histidine kinase PmHHK1 regulates polar growth, sporulation and cell wall composition in the dimorphic fungus Penicillium marneffei. Mycol. Res. 113:915–923 [DOI] [PubMed] [Google Scholar]
- 26. Posas F, Wurgler-Murphy SM, Maeda T, Witten EA, Thai TC, Saito H. 1996. Yeast HOG1 MAP kinase cascade is regulated by a multistep phosphorelay mechanism in the SLN1-YPD1-SSK1 “two-component” osmosensor. Cell 86:865–875 [DOI] [PubMed] [Google Scholar]
- 27. Saito H, Tatebayashi K. 2004. Regulation of the osmoregulatory HOG MAPK cascade in yeast. J. Biochem. 136:267–272 [DOI] [PubMed] [Google Scholar]
- 28. Wei H, Requena N, Fischer R. 2003. The MAPKK kinase SteC regulates conidiophore morphology and is essential for heterokaryon formation and sexual development in the homothallic fungus Aspergillus nidulans. Mol. Microbiol. 47:1577–1588 [DOI] [PubMed] [Google Scholar]
- 29. Lara-Rojas F, Sanchez O, Kawasaki L, Aguirre J. 2011. Aspergillus nidulans transcription factor AtfA interacts with the MAPK SakA to regulate general stress responses, development and spore functions. Mol. Microbiol. 80:436–454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Harris SD. 2011. Cdc42/Rho GTPases in fungi: variations on a common theme. Mol. Microbiol. 79:1123–1127 [DOI] [PubMed] [Google Scholar]
- 31. Boyce KJ, Hynes MJ, Andrianopoulos A. 2003. Control of morphogenesis and actin localization by the Penicillium marneffei RAC homolog. J. Cell Sci. 116:1249–1260 [DOI] [PubMed] [Google Scholar]
- 32. Boyce KJ, Schreider L, Andrianopoulos A. 2009. In vivo yeast cell morphogenesis is regulated by a p21-activated kinase in the human pathogen Penicillium marneffei. PLoS Pathog. 5:e1000678 doi:10.1371/journal.ppat.1000678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Todd RB, Andrianopoulos A. 1997. Evolution of a fungal regulatory gene family: the Zn(II)2Cys6 binuclear cluster DNA binding motif. Fungal Genet. Biol. 21:388–405 [DOI] [PubMed] [Google Scholar]
- 34. Nemecek JC, Wuthrich M, Klein BS. 2006. Global control of dimorphism and virulence in fungi. Science 312:583–588 [DOI] [PubMed] [Google Scholar]
- 35. Vargas-Pérez I, Sanchez O, Kawasaki L, Georgellis D, Aguirre J. 2007. Response regulators SrrA and SskA are central components of a phosphorelay system involved in stress signal transduction and asexual sporulation in Aspergillus nidulans. Eukaryot. Cell 6:1570–1583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Viaud M, Fillinger S, Liu W, Polepalli JS, Le Pecheur P, Kunduru AR, Leroux P, Legendre L. 2006. A class III histidine kinase acts as a novel virulence factor in Botrytis cinerea. Mol. Plant Microbe Interact. 19:1042–1050 [DOI] [PubMed] [Google Scholar]
- 37. Adams TH, Boylan MT, Timberlake WE. 1988. brlA is necessary and sufficient to direct conidiophore development in Aspergillus nidulans. Cell 54:353–362 [DOI] [PubMed] [Google Scholar]
- 38. Borneman AR, Hynes MJ, Andrianopoulos A. 2000. The abaA homologue of Penicillium marneffei participates in two developmental programmes: conidiation and dimorphic growth. Mol. Microbiol. 38:1034–1047 [DOI] [PubMed] [Google Scholar]
- 39. Hicks J, Lockington RA, Strauss J, Dieringer D, Kubicek CP, Kelly J, Keller N. 2001. RcoA has pleiotropic effects on Aspergillus nidulans cellular development. Mol. Microbiol. 39:1482–1493 [DOI] [PubMed] [Google Scholar]
- 40. Todd RB, Greenhalgh JR, Hynes MJ, Andrianopoulos A. 2003. TupA, the Penicillium marneffei Tup1p homologue, represses both yeast and spore development. Mol. Microbiol. 48:85–94 [DOI] [PubMed] [Google Scholar]
- 41. Borneman AR, Hynes MJ, Andrianopoulos A. 2002. A basic helix-loop-helix protein with similarity to the fungal morphological regulators, Phd1p, Efg1p and StuA, controls conidiation but not dimorphic growth in Penicillium marneffei. Mol. Microbiol. 44:621–631 [DOI] [PubMed] [Google Scholar]
- 42. Miller KY, Toennis TM, Adams TH, Miller BL. 1991. Isolation and transcriptional characterization of a morphological modifier: the Aspergillus nidulans stunted (stuA) gene. Mol. Gen. Genet. 227:285–292 [DOI] [PubMed] [Google Scholar]
- 43. Miller KY, Wu J, Miller BL. 1992. StuA is required for cell pattern formation in Aspergillus. Genes Dev. 6:1770–1782 [DOI] [PubMed] [Google Scholar]
- 44. Bugeja HE, Hynes MJ, Andrianopoulos A. 2010. The RFX protein RfxA is an essential regulator of growth and morphogenesis in Penicillium marneffei. Eukaryot. Cell 9:578–591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Cánovas D, Boyce KJ, Andrianopoulos A. 2011. The fungal type II myosin in Penicillium marneffei, MyoB, is essential for chitin deposition at nascent septation sites but not actin localization. Eukaryot. Cell 10:302–312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Li L, Xue C, Bruno K, Nishimura M, Xu J. 2004. Two PAK kinases genes, CHM1 and MST20, have distinct functions in Magnaporthe grisea. Mol. Plant Microbe Interact. 17:547–556 [DOI] [PubMed] [Google Scholar]
- 47. Cao C, Li R, Wan Z, Liu W, Wang X, Qiao J, Wang D, Bulmer G, Calderone R. 2007. The effects of temperature, pH, and salinity on the growth and dimorphism of Penicillium marneffei. Med. Mycol. 45:401–407 [DOI] [PubMed] [Google Scholar]
- 48. Bugeja HE, Hynes MJ, Andrianopoulos A. 2012. AreA controls nitrogen source utilisation during both growth programs of the dimorphic fungus Penicillium marneffei. Fungal Biol. 116:145–154 [DOI] [PubMed] [Google Scholar]
- 49. Cao C, Liu W, Li R. 2009. Penicillium marneffei SKN7, a novel gene, could complement the hypersensitivity of S. cerevisiae skn7 disruptant strain to oxidative stress. Mycopathologia 168:23–30 [DOI] [PubMed] [Google Scholar]
- 50. Jones DT, Taylor WR, Thornton JM. 1992. The rapid generation of mutation data matrices from protein sequences. Comput. Appl. Biosci. 8:275–282 [DOI] [PubMed] [Google Scholar]
- 51. Pongsunk S, Andrianopoulos A, Chaiyaroj SC. 2005. Conditional lethal disruption of TATA-binding protein gene in Penicillium marneffei. Fungal Genet. Biol. 42:893–903 [DOI] [PubMed] [Google Scholar]
- 52. Borneman AR, Hynes MJ, Andrianopoulos A. 2001. An STE12 homolog from the asexual, dimorphic fungus Penicillium marneffei complements the defect in sexual development of an Aspergillus nidulans steA mutant. Genetics 157:1003–1014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Cánovas D, Andrianopoulos A. 2006. Developmental regulation of the glyoxylate cycle in the human pathogen Penicillium marneffei. Mol. Microbiol. 62:1725–1738 [DOI] [PubMed] [Google Scholar]