Fig 4.
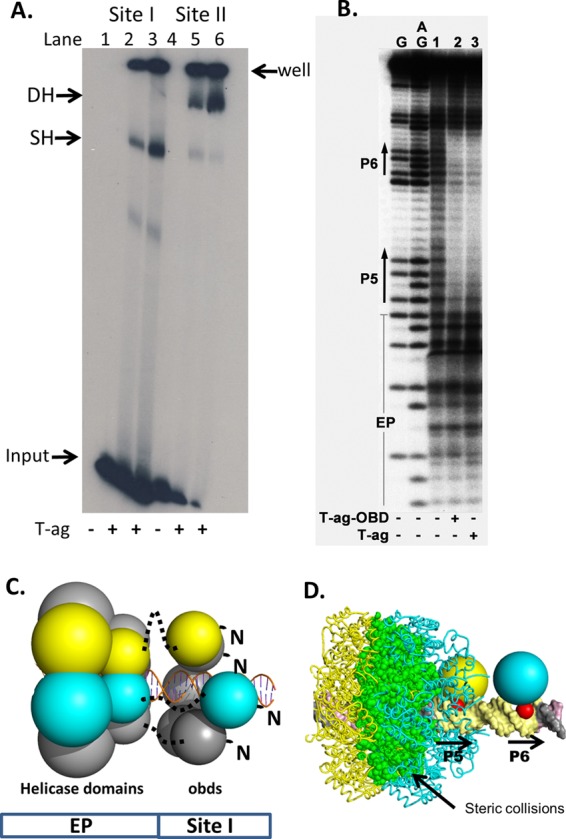
Full-length T-ag forms single hexamers on site I-containing DNA. (A) EMSA of full-length SV40 T-ag bound to a 59-bp oligonucleotide containing site I (lanes 2 and 3) or the 64-bp core origin oligonucleotide (lanes 5 and 6). The presence (+) or absence (−) of T-ag is indicated at the bottom. Lanes 2 and 5, 1.5 pmol T-ag; lanes 3 and 6, 3 pmol T-ag. The reaction products include single hexamers (SH) and double hexamers (DH). The DNA that did not enter the gel is labeled “well,” and the unbound DNA substrates are labeled “input.” (B) In situ footprinting of full-length T-ag and the OBD, when complexed to site I. The footprints were obtained using the gel retardation 1,10-phenanthroline–copper ion footprinting technique (57). The initial EMSAs were conducted with the previously described 47-bp site I + wt 30 oligonucleotide (50). Free DNA (i.e., DNA obtained from reactions conducted in the absence of protein and used as a control) is presented in lane 1. The locations of sequence features, including P5 and P6, are indicated. (C) Structure-based modeling of hexamers of the T-ag helicase and OBD domains on site I. The OBDs initially bound to P5 and P6 are shown as yellow and cyan, respectively. The OBDs are represented by single spheres, which are centered at the geometric center of mass, and the radius is approximately the radius of gyration of the domain (i.e., ∼17.5 Å). The helicase domains are represented by 2 spheres; those helicase domains connected to the initially bound OBDs are also shown as yellow and cyan. Dotted black lines represent the flexible linkers connecting the N terminus of the helicase domains to the C terminus of the OBDs. Finally, an idealized three-dimensional model of site I DNA, positioned along the 6-fold screw axis of the OBD spiral, is shown as a ribbon representation. (D) Molecular modeling studies indicating that two independent T-ag hexamers cannot form on P5 and P6. Two models of T-ag hexamers were constructed; one nucleated at P5 and one at P6. In the resulting model, significant collisions occur between the helicase domains (the collisions are shown in green).
