Abstract
Sindbis virus (SINV) infection of neurons results in nonfatal viral encephalomyelitis and provides a model system for understanding recovery from virus infection of the central nervous system (CNS). Infection is followed by clearance of infectious virus, a gradual decrease in viral RNA, and then long-term maintenance of low levels of viral RNA. Antibody to the E2 glycoprotein is important for virus clearance, and B cells enter the CNS along with CD4+ and CD8+ T cells during the early clearance phase. Antibody-secreting cells (ASCs) are present in the CNS and become enriched for SINV-specific ASCs. We have evaluated the factors within the CNS that facilitate continued local antibody production after infection. Expression of CXCL9, CXCL10, CCL1, CCL2, and CCL5 chemokine mRNAs increased early, and infiltrating B cells expressed CXCR3, CXCR5, and CCR7. The mRNAs for IL-10 and IL-21, cytokines important for B cell proliferation and differentiation, rose rapidly and remained elevated long after clearance of infectious virus. Active proliferation of B cells, as indicated by Ki-67 expression, continued for months. Bromodeoxyuridine (BrdU) labeling of proliferating cells showed that ASCs produced in the draining cervical lymph nodes during the early germinal center response were preferentially retained in the CNS. Sustained increase in B-cell-activating factor (BAFF) mRNA in the CNS and BAFF receptor expression by B cells coincided with the long-term maintenance of SINV-specific ASCs in the brain. We conclude that multiple changes in the brain microenvironment facilitate B-cell entry and support proliferation and differentiation and long-term survival of antiviral ASCs during recovery from alphaviral encephalomyelitis.
INTRODUCTION
Encephalitic alphaviruses infect neurons of the brain and spinal cord and are important causes of mosquito-borne encephalomyelitis in the Americas (1). Viral infection of neurons can have devastating consequences for the host, and recovery requires a rapid and effective immune response to clear infectious virus while protecting the sensitive, specialized, and nonregenerating neural tissue. Sindbis virus (SINV) infection of the central nervous system (CNS) of mice provides a model for understanding recovery from virus infection of neurons. Clearance of SINV is a noncytolytic process that is dependent on antibody (Ab) to the E2 glycoprotein (2). T-cell production of gamma interferon contributes to clearance of infectious virus from some populations of neurons (3), but viral RNA persists in the CNS long after recovery from the acute infection (4, 5).
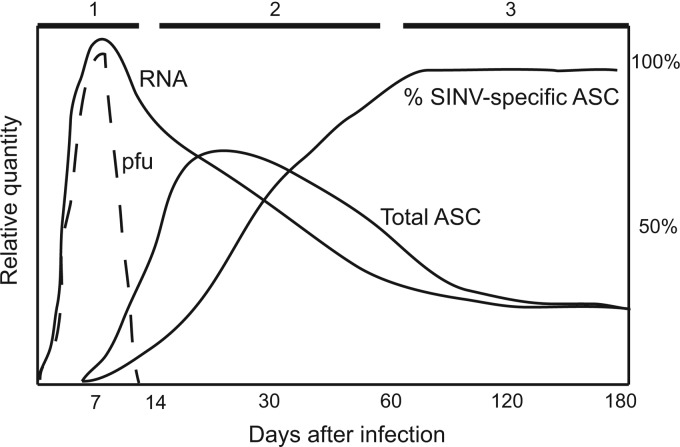
We have previously shown that SINV clearance from the CNS occurs in three phases (Fig. 1): clearance of infectious virus (days 3 to 7), clearance of most viral RNA (days 8 to 60), and maintenance of low levels of viral RNA and prevention of reactivation (beyond day 60) (6). During clearance of infectious virus (phase 1), inflammatory cells in the CNS are primarily CD8+ T cells and IgM Ab-secreting B cells (ASCs). During clearance of viral RNA (phase 2), CD4+ T cells are more abundant than CD8+ T cells, and B cells include IgG and IgA ASCs. During viral RNA persistence (phase 3), SINV-specific ASCs increase from 15% of total ASCs at day 14 to 90% by day 60 and secrete primarily IgG, suggesting specific retention of virus-specific ASCs in the infected brain.
Fig 1.
Schematic diagram of the three phases of brain virus clearance and ASC response after SINV infection. Phase 1, clearance of infectious virus (PFU); phase 2, infiltration of ASCs that are increasingly enriched for cells producing SINV-specific IgG and decrease in viral RNA to low levels; phase 3, maintenance of SINV-specific ASCs and low levels of viral RNA. The diagram is based on data from Metcalf and Griffin (6).
The presence of antiviral ASCs in the CNS has been observed following other neurotropic virus infections, such as those caused by measles virus (7–9), West Nile virus (10), rabies virus (11), Semliki Forest virus (12, 13), Theilers murine encephalomyelitis virus (14), and the JHM strain of mouse hepatitis virus (JHMV) (15, 16). There is also substantial evidence that entry and retention of ASCs in the CNS are important for virus clearance and prevention of reactivation (17, 18). ASCs retained in the CNS in response to viral infection have variously been identified as fully differentiated, nondividing plasma cells (PCs) or less mature plasmablasts (PBs) (6, 10, 14, 16).
In the periphery, after recovery from viral infection, PCs are retained primarily in the bone marrow, where they occupy special niches that promote long-term survival and continued Ab secretion (19, 20). In the bone marrow, PCs are in contact with reticular stromal cells that express chemotactic, survival, and differentiation factors such as interleukin-5 (IL-5), IL-6, vascular cell adhesion molecule 1 (VCAM-1), tumor necrosis factor (TNF), B-cell-activating factor of the TNF family (BAFF), and CXCL12. In tissue sites of infection, long-term maintenance of local Ab production requires either entry and retention of long-lived PCs, continued entry of ASCs from the periphery, turnover of PBs in situ, or continued replacement of ASCs from memory B cells residing in the tissue.
Most studies that have evaluated long-term B-cell survival in the CNS have focused on the human autoimmune demyelinating disease multiple sclerosis. Characteristic features of this disease are the presence of oligoclonal immunoglobulin bands in the cerebrospinal fluid and persistence of clonally expanded B cells and PCs in the CNS (21). Chemokines and cytokines that are increased in the brains of multiple sclerosis patients foster entry (CXCL9/CXCL10), follicle formation and differentiation (CXCL13), and long-term survival (BAFF) of B cells (22–26). However, little is known about molecular cues in the brain microenvironment involved in recruitment and retention of ASCs after virus infection of the CNS.
Our previous studies of the B cells in the CNS of mice infected with SINV showed continued presence of both PBs and memory B cells but not PCs, suggesting the need for renewal of CNS ASCs to maintain local Ab production (6). In the present study, we have examined the role of the brain microenvironment in mediating recruitment and retention of ASCs in the CNS of mice with SINV infection of neurons and show that infection induces a brain microenvironment that supports the entry, proliferation, differentiation, and long-term survival of SINV-specific ASCs.
MATERIALS AND METHODS
Mice and virus infection.
Female 4- to 6-week-old C57BL/6J mice were obtained from Jackson Laboratory (Bar Harbor, ME). Mice were infected by intracerebral inoculation with 1,000 PFU of TE, a recombinant strain of SINV (27). Control mice were inoculated with phosphate-buffered saline (PBS) that, as was previously shown, does not induce an inflammatory response (6). All procedures were performed in compliance with protocols approved by the Johns Hopkins University Animal Care and Use Committee.
Chemokine and cytokine qRT-PCR.
RNA was isolated using a Qiagen RNAeasy Lipid Tissue Mini Kit. For preparation of cDNA, 5 μg of RNA was amplified using random hexamers from a High Capacity cDNA Reverse Transcription Kit (Applied Biosystems). Quantitative real-time PCR (qRT-PCR) was performed using 2.5 μl of cDNA, TaqMan Universal PCR Master Mix, and 20× Gene Expression Assays (primer and probe mixture) from Integrated DNA Technologies (CXCL1 and CXCL2) or Applied Biosystems (all others). Comparative threshold cycle (CT) values were generated using day 0 tissue samples as the calibrator/control and tissues from SINV- and PBS-infected mice as experimental samples. Values were normalized to endogenous mouse glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA. PCRs (40 cycles) were performed in triplicate on a 7500 Fast Real-Time PCR System and analyzed by relative quantitation using Sequence Detector software, version 1.4 (Applied Biosystems).
Isolation of mononuclear cells.
Mononuclear cells were isolated from brains and cervical lymph nodes (CLNs) as previously described (6). Briefly, brains (pooled from 4 to 6 mice) were homogenized using a Neural Tissue Dissociation kit with trypsin along with gentleMACS C-Tubes and Dissociator (Miltenyi Biotec). The dissociated tissue was incubated at 37°C with DNase and collagenase, followed by filtration through a 70-μm-pore-size cell strainer washed with cold 0.5% bovine serum albumin-Hanks balanced salt solution without Ca2+ and Mg2+ (BSA-HBSS). Cells were pelleted and resuspended in cold 0.9 M sucrose in HBSS with Ca2+ and Mg2+ to separate the cells from myelin. After centrifugation at 850 × g for 10 min with slow braking, the cell pellet was washed in cold HBSS with Ca2+ and Mg2+. CLNs (pooled from 4 to 6 mice) were homogenized in cold PBS–2 mM EDTA–0.5% BSA (PEB) using gentleMACS C-Tubes and Dissociator. Brain and CLN cell suspensions were filtered through a 70-μm-pore-size strainer, pelleted, and resuspended in hypotonic ammonium chloride to lyse contaminating red blood cells. The lysis reaction was quenched with the addition of either BSA-HBSS (brain) or PEB (CLN) before cells were pelleted, resuspended in PEB, and counted.
Flow cytometry.
Staining was performed in a 96-well round-bottom plate with 106 cells per well. Dead cells were distinguished using a Live/Dead Fixable Dead Cell Stain Kit (Invitrogen). Fcγ receptors were blocked using anti-mouse CD16/CD32 (Miltenyi Biotec) diluted in PEB. Cells were stained with Abs to surface markers for 30 min on ice, followed by intracellular staining using a BD Cytofix/Cytoperm kit. Abs were diluted 1:100 in PEB for surface staining and in BD Perm/Wash buffer for intracellular staining. Monoclonal Abs from BD Biosciences were against CD19 (clone 1D3), CD3 (clone 17A2), Ki-67 (clone B56), IL-21 receptor (IL-21R) (clone 4A9), CXCR4 (clone 2B11/CXCR4), and CXCR5 (clone 2G8). Monoclonal Abs from eBioscience were against CCR5 [clone HM-CCR5(7A4)], CCR7 (clone 4B12), CXCR3 (clone CXCR3-173), BAFF receptor (BAFFR) (clone 7H22-E16), and TACI (clone 8F10-3). Cells were analyzed on a BD FACSCanto II with FACSDiva software, version 6. Initial gating of the stained mononuclear cell populations was as previously described (6). Statistical analysis was performed with FlowJo software, version 9 (Treestar, Ashland, OR).
In vivo BrdU labeling.
SINV- and PBS-inoculated mice were given bromodeoxyuridine (BrdU; Sigma) in their drinking water (0.8 mg/ml) for 4 days. Labeling after infection was performed during the following periods: days 4 to 7, days 7 to 10, and days 11 to 14. BrdU-treated water was changed every other day. Mononuclear cells were isolated from the brain and CLNs at 1 day, 1 month, 2 months, and 4 months after labeling (pooled from 4 mice). Immunofluorescent staining of incorporated BrdU was performed using a BD BrdU fluorescein isothiocyanate (FITC) flow kit. Briefly, staining of surface markers was performed on ice using cold PEB. After a washing step, cells were fixed and permeabilized with BD Cytofix/CytoPerm buffer and BD Cytoperm Plus buffer. Cells were washed with BD Perm/Wash buffer followed by incubation with DNase (30 μg of DNase/106 cells) for 1 h at 37°C to expose BrdU epitopes. After cells were washed with BD Perm/Wash buffer, they were incubated with FITC-conjugated anti-BrdU Ab for 20 min on ice, resuspended in PBS–0.1% BSA and analyzed on a BD FACSCanto II with FACSDiva software. Statistical analysis was performed with FlowJo software. Staining of cells was done in duplicate for each time point. The mean PBS values (labeling background) were subtracted from corresponding SINV time points, and resulting values were plotted as means ± standard errors of the means (SEM).
Immunohistochemistry.
SINV- and PBS-inoculated mice at 7, 10, 14, 26, 120, and 180 days after inoculation were perfused with cold PBS followed by ice cold fresh 10% formaldehyde in PBS. Brains were removed and cut into 2-mm coronal slices using an adult Mouse Brain Slicer (Zivic Instruments), and slices were placed into cassettes and fixed in formalin for 48 h at 4°C. Slices were washed in PBS for 24 h at 4°C and embedded in paraffin. For staining, sections from three levels were baked at 58°C for 30 min and then rehydrated. For antigen retrieval, slides were boiled in 10 mM sodium citrate, pH 6.0, for 9 min. Endogenous peroxidase was blocked with 3% H2O2 for 10 min before incubation with blocking buffer (10% normal goat serum, 0.05% Tween 20 in PBS). Slides were stained with polyclonal rabbit anti-Ki-67 (1:200; Abcam), biotinylated goat anti-rabbit IgG (1:300; Vector), and avidin-biotin complex (Vectastain Elite ABC Kit) developed with 3,3′-diaminobenzidine (Vector). Slides were then blocked using an Avidin/Biotin Blocking Kit (Vector) and stained with rat monoclonal (RA3-6B2) anti-CD45R/B220 (1:200; Abcam) and biotinylated rabbit anti-rat IgG (1:300; Vector) developed with VIP substrate (Vector). Slides were dehydrated and mounted in Permount. For each section, the percentage of B220+ B cells that were Ki-67+ was determined.
RESULTS
CNS chemokine mRNA expression.
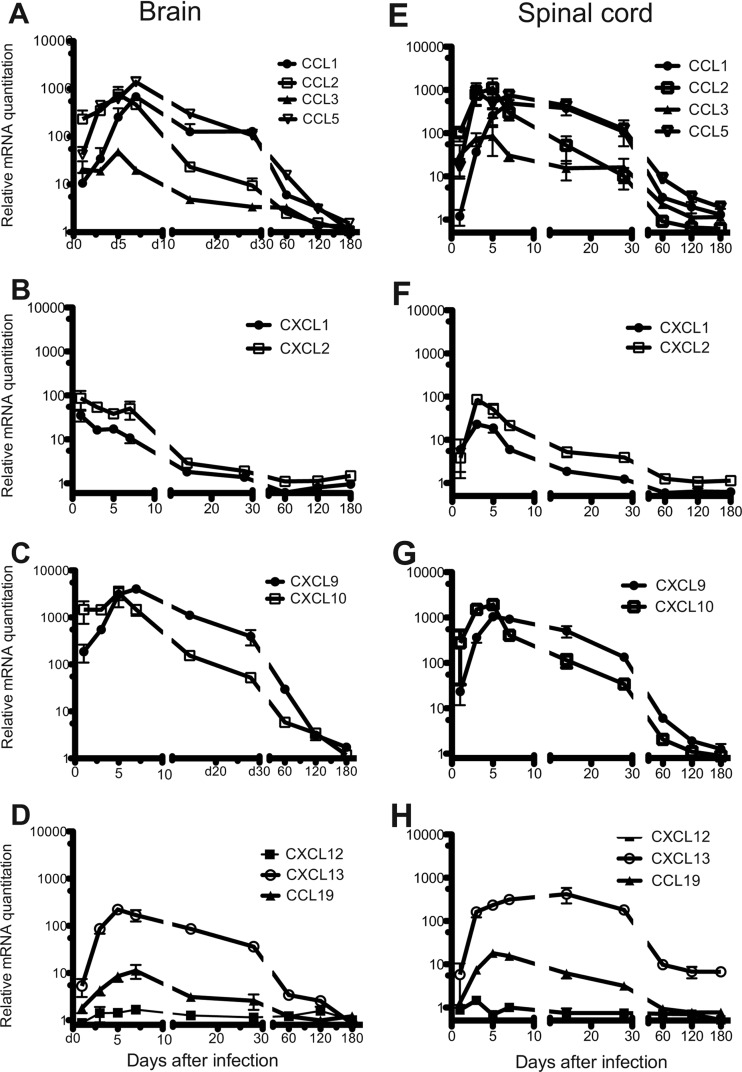
Migration of leukocytes into the CNS in response to viral infection is guided by local production of inflammatory chemokines such as CXCL9, CXCL10, CCL1, CCL2, CCL3, and CCL5 (28, 29). To characterize the CNS chemokine environment induced by SINV infection, chemokine mRNAs were assessed by qRT-PCR (Fig. 2). The mRNAs of CCL1, CCL2, CCL5, CXCL9, and CXCL10 were highly upregulated in both the brain (Fig. 2A and C) and spinal cord (Fig. 2E and G), peaking at 5 to 7 days after infection. CCL3 mRNA (Fig. 2A and E) and mRNAs of CXCL1 and CXCL2 (Fig. 2B and F), chemokines involved in neutrophil trafficking (30), increased during the same time period but reached lower maximal levels. All upregulated chemokine mRNAs peaked before CD3+ T cells and CD19+ B cells reached maximal levels 10 days after infection (6). Chemokine mRNA levels declined between 10 and 60 days, followed by a continued slow decrease to near baseline 6 months after infection.
Fig 2.
CNS expression of chemokine mRNAs involved in leukocyte trafficking. C57BL/6 mice were inoculated intracerebrally with 1,000 PFU of SINV. Brains and spinal cords were analyzed for the mRNAs of chemokines CCL1, CCL2, CCL3, and CCL5 (A and E), CXCL1 and CXCL2 (B and F), CXCL9 and CXCL10 (C and G), and CCL19, CXCL12, and CXCL13 (D and H). Each point is the mean ± SEM of three mice, except for CCL1 for which there are two mice for days 1 to 28. Relative levels were normalized to endogenous GAPDH mRNA expression.
The mRNAs of CXCL13 and CCL19, homeostatic chemokines involved in lymphoid follicle formation and in trafficking of undifferentiated B cells (31), also increased in response to infection, with maximal levels in the brain 5 to 7 days after infection (Fig. 2D and H). The mRNA expression of CXCL12, a chemokine involved in PC migration and survival (32), did not change in response to SINV infection (Fig. 2D and H).
Chemokine receptors on infiltrating B cells.
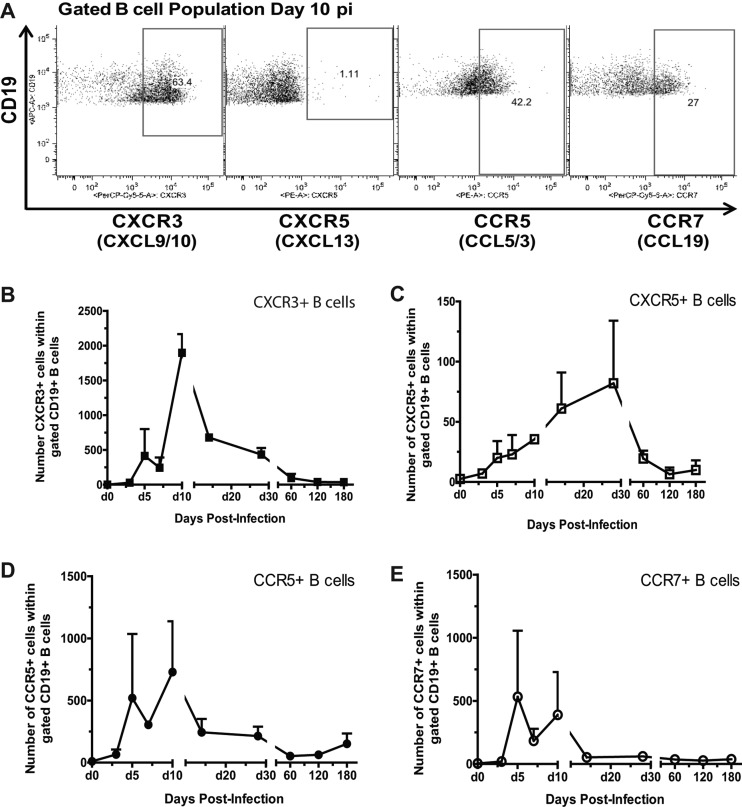
Using flow cytometry, the expression levels of CXCR3 (receptor for CXCL9 and CXCL10), CXCR5 (receptor for CXCL13), CCR5 (receptor for CCL3 and CCL5), and CCR7 (receptor for CCL19) were analyzed on infiltrating B cells to determine whether these receptor-ligand pairs may be involved in B-cell homing to SINV-infected brain (Fig. 3A). CXCR3 was the main receptor detected on infiltrating B cells for up to 2 months after infection (Fig. 3B). A smaller proportion of infiltrated B cells expressed CCR5 and CCR7, which peaked at days 5 and 10 after infection (Fig. 3D and E). A small population of CXCR5+ B cells was maximal later, at day 28 after infection (Fig. 3C). By 60 days, few cells expressed any of the chemokine receptors examined. These observations suggest that CXCR3 ligands CXCL9 and CXCL10 are major contributors to B-cell recruitment into the CNS in response to SINV infection, with CCR5/CCL5 and CCR7/CCL19 as more minor additional contributors. Furthermore, most recruitment occurs during the first 10 days and decreases after the clearance of infectious virus.
Fig 3.
The expression of chemokine receptors on infiltrated B cells. C57BL/6 mice were inoculated intracerebrally with 1,000 PFU of SINV. Mononuclear cells isolated from pooled brains of infected mice (n = 6) were gated as previously described (6) and analyzed for CD19 and chemokine receptor expression by flow cytometry. (A) The flow cytometry gating scheme for detecting CXCR3+, CXCR5+, CCR5+, and CCR7+ B cells within the CD19+ population. The gated numbers of B cells expressing CXCR3+ (B), CXCR5+ (C), CCR5+ (D), and CCR7+ (E) within the gated CD19+ population in SINV-infected brain are shown. Each point is the mean ± SEM of two experiments. p.i., postinfection.
Proliferation of infiltrated B cells in the CNS.
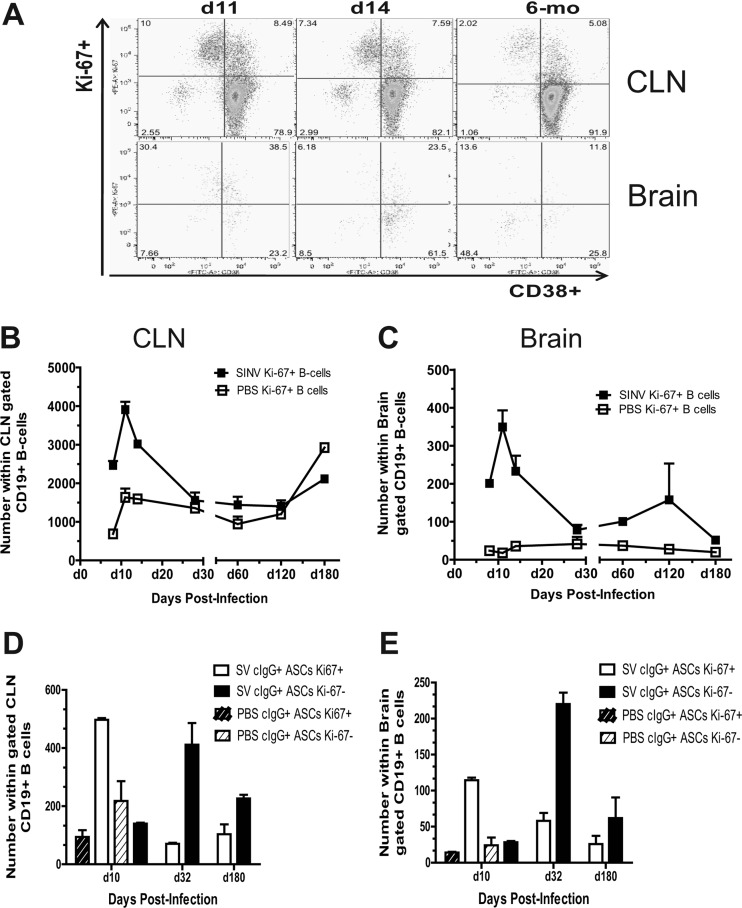
Previously, we have shown that infiltrated B cells have a PB phenotype (CD19+ CD38+ CD138− cytoplasmic IgG positive [cIgG+]) (6). To determine whether these cells are proliferating within the CNS during the clearance of viral RNA, we identified by flow cytometry the proportion of B cells expressing Ki-67 (Fig. 4A), a protein present in the nuclei of proliferating or recently proliferated cells but not in quiescent or resting cells (33). In the CLNs, Ki-67+ B cells reached maximal levels at 11 days after infection, during the time of the germinal center (GC) response (Fig. 4B). A portion of these cells appear to be GC B cells which have downregulated their CD38 expression (CD19+ CD38− Ki-67+) (Fig. 4A, top). In the brain, Ki-67+ B cells also reached maximal levels at 11 days after infection, during the time of peak infiltration into the brain (Fig. 4A, bottom, and C). The numbers of Ki-67+ B cells decreased to low but detectable levels, representing approximately 25% of CD19+ B cells in brain tissue after the SINV-induced inflammatory response waned 1 month after infection.
Fig 4.
The number of proliferating B cells in the brain. Mononuclear cells isolated from pooled brains of SINV- and PBS-inoculated mice (n = 4) were analyzed for CD19, CD38, and Ki-67 expression. (A) The flow cytometry gating scheme for detecting Ki-67+ B cells in the CLNs and brain. The numbers of Ki-67+ B cells in the CLNs (B) and brain (C) are shown. The numbers of Ki-67+ or Ki-67−B cells expressing intracellular cIgG within the gated CD19+ population in the CLNs (D) and brain (E) are plotted. Staining of cells was done in duplicate for each time point. Each point is the mean ± SEM of one experiment.
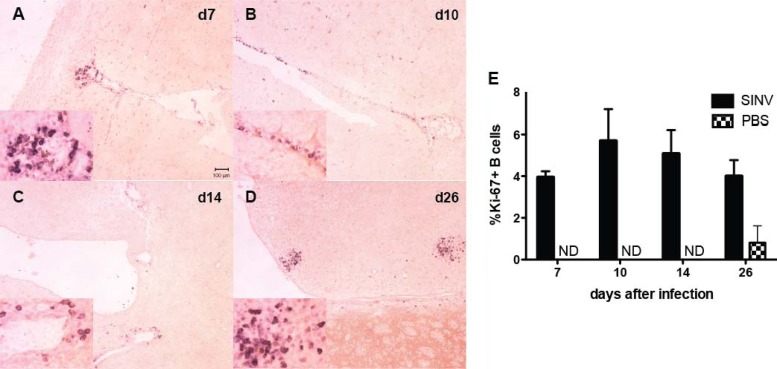
During differentiation, PC and PB ASCs downregulate surface immunoglobulins while increasing production of intracellular immunoglobulin (34, 35). Memory B cells have low levels of intracellular immunoglobulin until expansion and differentiation into ASCs (36). To determine if ASCs were proliferating in the brain, we analyzed Ki-67+ B cells for the expression of intracellular cytoplasmic IgG (cIgG). At 10 days after infection, a majority of cIgG+ ASCs were Ki-67+ in both CLNs and brain, while a month or more after infection most cells, but not all, were Ki-67− (Fig. 4D and E). Substantial numbers of Ki-67+ B cells, often in periventricular clusters, could be identified by immunohistochemistry in brain at 7, 10, 14, and 26 days after infection (Fig. 5). Single scattered cells were identified 4 and 6 months after infection. Thus, these data suggest that a population of proliferating ASCs is present in the brain after the clearance of infectious virus.
Fig 5.
Immunohistochemical identification of Ki-67+ B cells in brain. Formalin-fixed, paraffin-embedded brains from SINV-inoculated (n = 3) and PBS-inoculated (n = 2) mice were sectioned at three levels and stained for Ki-67 and B220 to identify recently proliferated B cells. Representative sections are shown from 7 days (d7; A), 10 days (B), 14 days (C), and 26 days (D) after infection. (E) Averages ±SEM of the percent Ki-67+ B cells per section. ND, none detected.
The life span of infiltrated B cells.
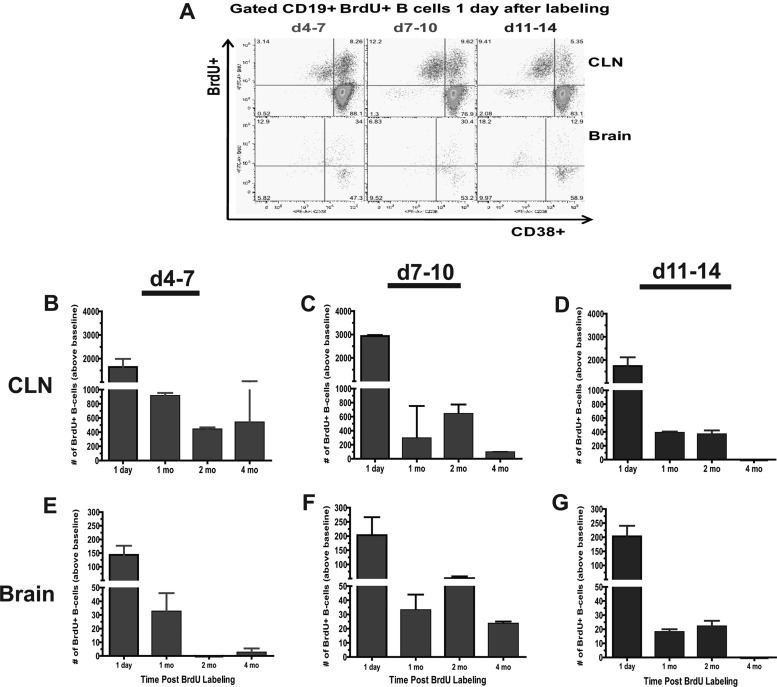
The presence of SINV-specific ASCs months after clearance could be due to continuous infiltration from the periphery and/or persistence of ASCs that entered the CNS during the acute immune response. To determine the life span of B cells in the brain, PBS-inoculated and SINV-infected mice were given BrdU in their drinking water to mark B cell “birthdays” during three 4-day time periods: the extrafollicular response (days 4 to 7), early GC response (days 7 to 10), and late GC response (days 11 to 14) (Fig. 6). In the CLNs, the largest numbers of B cells became BrdU positive (BrdU+) during the early GC response days 7 to 10 after infection (Fig. 6C). GC B cells were also identified within the gated BrdU+ B cells by lack of expression of CD38 (Fig. 6A). Larger numbers of proliferating GC B cells (CD19+ CD38− BrdU+) were detected in CLN during the early GC response than in the late GC response (Fig. 6A top). Four months after labeling, BrdU+ B cells labeled during the extrafollicular response and early GC response were still detected above baseline in the CLNs (Fig. 6B and C).
Fig 6.
The life span of infiltrated B cells in the brain. Mononuclear cells isolated from pooled brains of SINV- and PBS-inoculated mice (n = 4) were analyzed for CD19, CD38, and BrdU expression. (A) The flow cytometry gating scheme for detecting BrdU+ B cells in the CLNs and brain 1 day after labeling. Labeling periods were days 4 to 7, days 7 to 10, and days 11 to 14. The numbers of BrdU+ B cells above baseline for CLNs (B, C, and D) and brain (E, F, and G) within the gated CD19+ population are plotted. Staining of cells was done in duplicate for each time point. The mean PBS value (background labeling) was subtracted from each corresponding SINV time point. Each point is the mean ± SEM of one experiment.
B cells labeled during each of the immune phases were detected in the brain (Fig. 6A and E to G). These observations suggest that B cells labeled during the extrafollicular response, as well as during the GC response, can exit the CLNs and migrate to the brain. However, these early extrafollicular cells were not detected at 2 and 4 months after infection and so appear to be short-lived outside the CLNs (Fig. 6E). Early GC-derived BrdU+ B cells were still detected in the brain 2 and 4 months after labeling (Fig. 6F), while late GC-derived cells were detected at 2, but not 4, months. When BrdU labeling was conducted at 180 days after infection, very few B or T cells became labeled (data not shown). Thus, these data suggest that GC-derived B cells that infiltrate the CNS during the acute phase of the response to infection are maintained in the brain months after clearance of infectious virus.
CNS expression of cytokines involved in B-cell proliferation and differentiation.
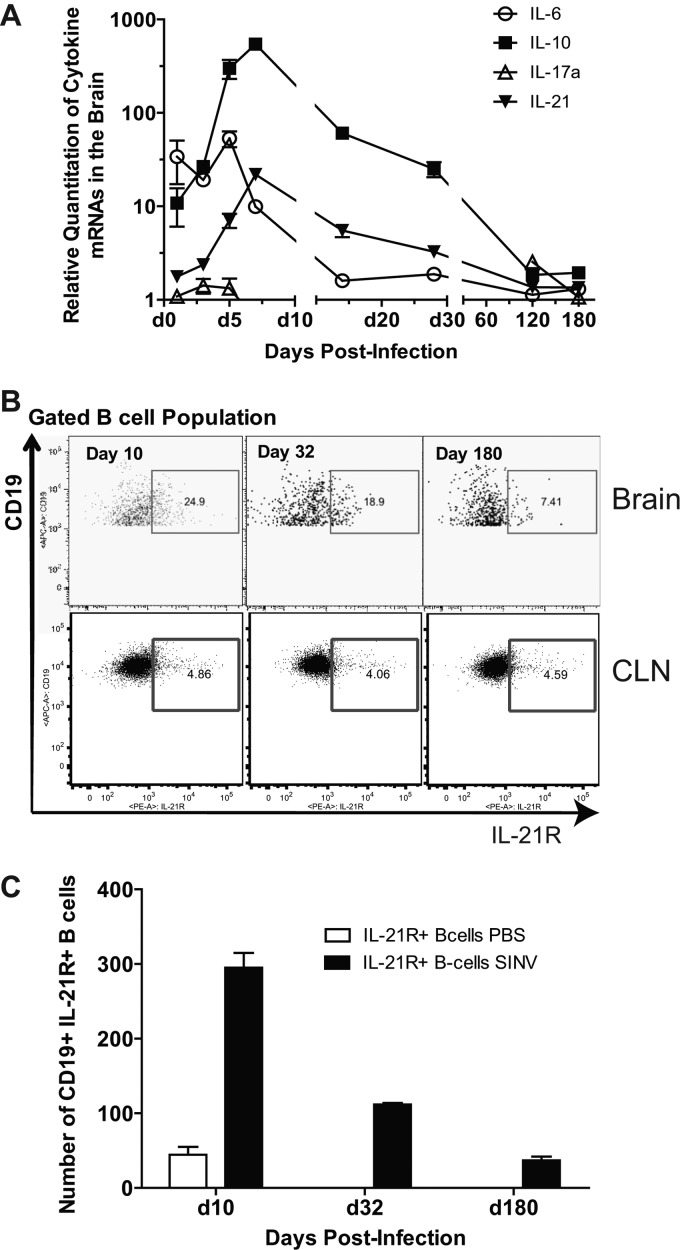
Previous enzyme-linked immunospot (ELISpot) assay data showed that SINV-specific ASCs continue to increase and differentiate (e.g., into IgA ASCs) after establishing residence in the brain (6). This conclusion is supported by Ki-67 data that showed that B cells continue to proliferate in the brain after clearance of infectious virus (Fig. 4C and 5). To identify factors in the CNS microenvironment that may enhance B-cell proliferation, differentiation, and survival, we analyzed IL-6, IL-10, and IL-21 mRNA expression in SINV-infected brains (37–40) (Fig. 7A). IL-6 mRNA was moderately upregulated from 1 to 7 days, with a peak at 5 days after infection. IL-10 mRNA was highly upregulated beginning at 1 day, peaking at 7 days, and continuing above baseline for at least 4 weeks after infection. The mRNA of IL-17A, a pleiotropic cytokine that acts on multiple cell types to enhance the production of proinflammatory molecules including IL-6 (41), showed no change.
Fig 7.
CNS expression of cytokines involved in B-cell survival and differentiation. C57BL/6 mice were inoculated intracerebrally with 1,000 PFU of SINV. (A) Relative quantitation values in the brain and spinal cord for mRNAs of IL-6, IL-10, IL-21, and IL-17a. Each point is the mean ± SEM of three mice. Relative levels were normalized to endogenous GAPDH mRNA expression. (B) The flow cytometry gating scheme for detecting IL-21R+ B cells within the CD19+ population. (C) The gated cell number of IL-21R+-expressing B cells in SINV- and PBS-inoculated brain. Each point is the mean ± SEM of one experiment.
The mRNA of IL-21, a strong inducer of memory B cells, PC differentiation, and isotype switching, increased more slowly, with maximal levels at 7 days and sustained elevation through day 28 after infection. Using flow cytometry, the expression of IL-21 receptor (IL-21R) was analyzed on infiltrated B cells (Fig. 7B). IL-21R+ B cells were present in the brain, reaching maximal levels at day 10 after infection, with expression on 25% of infiltrated B cells and continued expression on a subset of B cells for at least 6 months (Fig. 7B and C). These observations suggest that the brain provides a microenvironment that supports in situ proliferation and differentiation of ASCs.
CNS expression of B-cell survival factors.
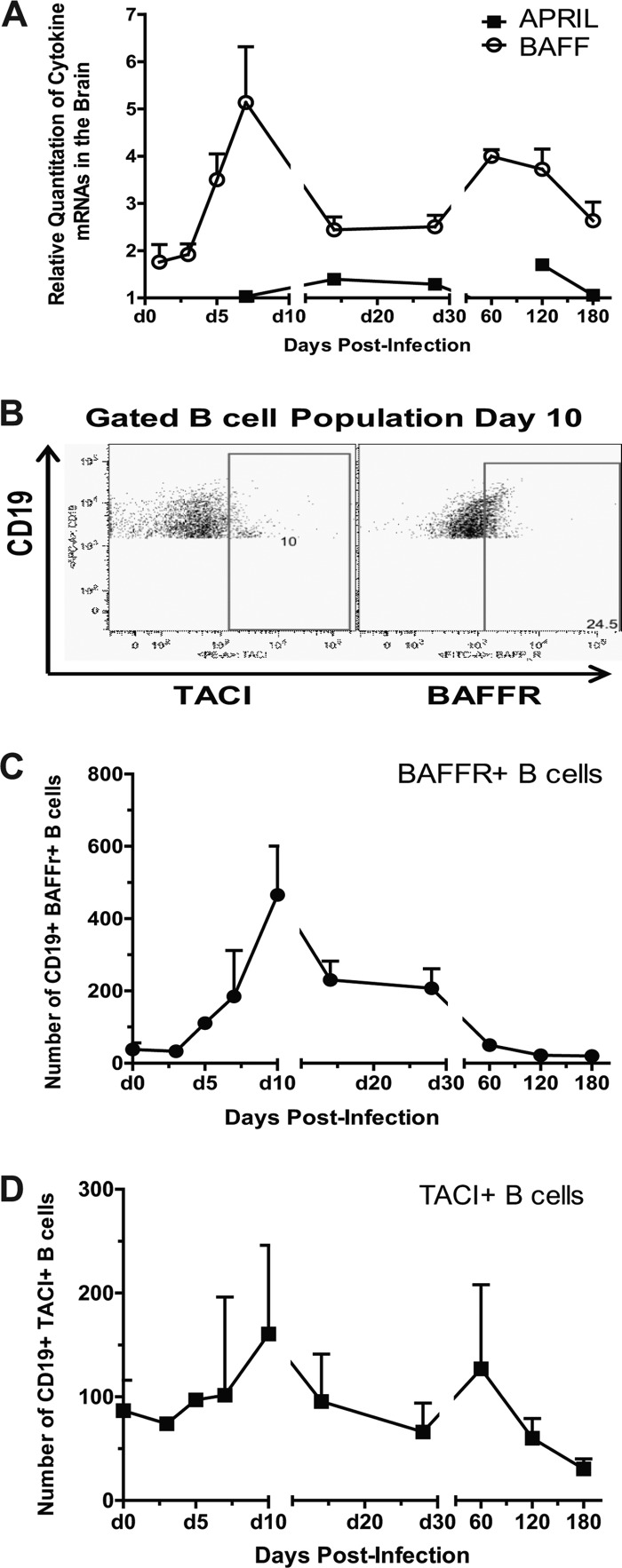
SINV-specific ASCs are present in the brain 6 months after infection (Fig. 1) (6), and BrdU+ B cells were detected in the brain for at least 4 months after being labeled in the periphery 7 to 10 days after infection (Fig. 6F). Both BAFF and APRIL are essential for the long-term maintenance of PCs in the bone marrow (19, 42), and long-term maintenance of ASCs in the brain suggests the presence of survival factors in the CNS. In the normal human brain, both BAFF and APRIL are constitutively produced by astrocytes and are strongly upregulated in multiple sclerosis lesions (24, 43). In SINV-infected brain, BAFF mRNA was upregulated by 5 days and peaked at 7 days, with continued expression above baseline for at least 6 months after infection (Fig. 8A). APRIL mRNA showed little change in expression (Fig. 8A).
Fig 8.
CNS expression of cytokines involved in B-cell survival. C57BL/6 mice were inoculated intracerebrally with 1,000 PFU of SINV. (A) The relative quantitation values in the brain and spinal cord for mRNAs of BAFF and APRIL. Each point is the mean value ± SEM of three mice. Relative levels were normalized to endogenous GAPDH mRNA expression. (B) The flow cytometry gating scheme for detecting BAFFR- and TACI-positive B cells within the CD19+ population. (C) The gated cell number of BAFFR- and TACI-expressing B cells in SINV-infected brain. Each point is the mean ± SEM of two experiments (one experiment for day 5).
Using flow cytometry, the expression of BAFF receptors BAFFR and TACI was analyzed on infiltrated B cells (Fig. 8B). A higher proportion of infiltrated B cells expressed BAFFR, which peaked 10 days after infection (Fig. 8C), than TACI, which showed only a slight increase from baseline (Fig. 8D). Thus, these data provide further evidence that the brain can provide a microenvironment that supports long-term survival of ASCs.
DISCUSSION
These studies have shown that changes in the brain microenvironment in response to SINV infection facilitate B-cell entry and support proliferation, differentiation, and long-term survival of antiviral ASCs. These changes included increases in expression of chemokine CXCL9, CXCL10, CCL1, CCL2, and CCL5 mRNAs with concomitant infiltration of B cells expressing CXCR3, CXCR5, and CCR7. B-cell proliferation was most marked early after entry, but ASC proliferation continued for weeks, and the mRNAs for IL-10 and IL-21, cytokines important for B-cell proliferation and differentiation, rose rapidly and remained elevated after clearance of infectious virus. ASCs produced in the CLNs 7 to 10 days after infection during the early GC response were preferentially retained in the CNS after viral RNA had decreased to low levels. BAFF mRNA was upregulated in the brain for at least 6 months after infection, coinciding with the long-term maintenance of SINV-specific ASCs and local Ab production after recovery.
Chemokine CXCL9 and CXCL10 mRNAs peaked during the time of maximal trafficking of CD8+ T cells but before the maximal accumulation of CD4+ T cells and CD19+ B cells at 10 days after infection. This was followed by a steady decline to baseline by 6 months after infection that correlates with the steady decline in total ASCs and T cells. Multiple cells in the CNS, including neurons, can produce these chemokines (44, 45). PBs express CXCR3 and home to CXCL9 and CXCL10 in vitro (46), and our preliminary data have shown that, in response to SINV, infiltrating ASCs (CD19+ cIgG+) and memory B cells (CD19+ surface IgG+ [sIgG+]) express CXCR3. These data are consistent with studies of JHMV-infected mice that showed sustained upregulation of CXCL9 and CXCL10 mRNAs in the CNS (16) and show an 80% reduction of total and virus-specific ASC migration to the CNS in CXCR3−/− mice (18). Therefore, this chemokine/chemokine receptor combination appears to be particularly important for B-cell entry into the CNS.
The detection of proliferating Ki-67+ B cells in the brain supports ELISpot assay data that show increasing numbers of SINV-specific ASCs after the clearance of infectious virus. In the brain, the highest percentage of Ki-67+ immune cells occurred during the acute phase of the immune response, mirroring the CLNs, but small numbers of Ki-67+ B cells were detected for at least 6 months after infection. Because the blood-brain barrier is less permeable after the inflammatory response wanes and there is a steady decline in production of inflammatory chemokines in the CNS, it is unlikely that these proliferating cells are entering from the periphery. Furthermore, in vivo labeling with BrdU during the acute immune response implies that early GC-derived B cells can persist in the brain for months. This suggests that active in situ proliferation of B cells in the CNS can maintain local production of antiviral Ab during the clearance of viral RNA and prevent reactivation.
IL-6 and IL-10 enhance B-cell survival, proliferation, differentiation, and Ab production (37) and upregulate Blimp-1 and XBP-1, transcription factors that are involved in PC differentiation (38). IL-21 is a strong inducer of B-cell proliferation, differentiation, and isotype switching (39, 40). The upregulation of these cytokine mRNAs provides supporting evidence that the brain is capable of providing an environment that facilitates proliferation and differentiation of SINV-specific ASCs. These factors remained elevated in the brain during the critical time of virus RNA clearance when the local Ab response is increasingly SINV specific. In GCs, IL-21 is produced by CD4+ follicular helper T cells (47). CD4+ T cells in the CNS also produce IL-21 (48), and T cells shift from CD8+ to CD4+ T cells after the clearance of infectious virus. Therefore, SINV-specific ASCs are likely maintained through interaction with SINV-specific IL-21-producing CD4+ T cells that provide a signal for further differentiation and retention while nonspecific B-cells exit or undergo apoptosis. The upregulation of BAFF provides the signal needed for long-term survival of these differentiated cells. Astrocytes in the CNS constitutively produce BAFF, and microglial cells secrete BAFF in vitro (24, 49). In JHMV infection, the long-term maintenance of ASCs in the CNS after clearance of infectious virus and during persistence of viral RNA in glial cells coincides with astrocyte production of BAFF mRNA (16, 48).
The formation of GC follicles in the brain would be another mechanism for maintaining SINV-specific ASCs. Studies of multiple sclerosis have detected the formation of ectopic lymphoid follicle structures that are thought to facilitate the differentiation of centroblasts, centrocytes, and memory B cells into short-lived PBs in the CNS (50). The expression of CXCL13 and CXCL12 is essential for follicle formation in the CNS (22, 23). CXCL13 mRNA was not elevated in the brains of JHMV-infected mice (48). In response to SINV, CXCL13 and CCL19 mRNAs were induced in the brain, but CXCR5+ and CCR7+ B cells did not appear to be infiltrating the brain, and other investigators have shown that CXCL13−/− mice infected with SINV have normal B-cell infiltration and virus clearance (51). This observation, along with limited detection of B cells with GC markers (6), implies that lymphoid follicles are not forming in the brain and are not a source of persisting ASCs.
In conclusion, changes in the brain microenvironment in response to SINV infection facilitate B-cell entry and support proliferation, differentiation, and long-term survival of antiviral ASCs. Local production of Ab is likely to be important for clearance of viral RNA from neurons and for prevention of virus reactivation.
ACKNOWLEDGMENTS
This work was supported by research grant R01 NS038932 (D.E.G.) and training grants T32 AI007417 (T.U.M.) and T32 OD011089 (V.K.B.) from the National Institutes of Health and a scholarship from the Anandamahidol Foundation of Thailand (V.N.).
Footnotes
Published ahead of print 19 December 2012
REFERENCES
- 1. Calisher CH. 1994. Medically important arboviruses of the United States and Canada. Clin. Microbiol. Rev. 7:89–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Levine B, Hardwick JM, Trapp BD, Crawford TO, Bollinger RC, Griffin DE. 1991. Antibody-mediated clearance of alphavirus infection from neurons. Science 254:856–860 [DOI] [PubMed] [Google Scholar]
- 3. Binder G, Griffin DE. 2001. Interferon-γ-mediated site specific clearance of alphavirus from CNS neurons. Science 293:303–306 [DOI] [PubMed] [Google Scholar]
- 4. Levine B, Griffin DE. 1992. Persistence of viral RNA in mouse brains after recovery from acute alphavirus encephalitis. J. Virol. 66:6429–6435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tyor WR, Wesselingh S, Levine B, Griffin DE. 1992. Long term intraparenchymal Ig secretion after acute viral encephalitis in mice. J. Immunol. 149:4016–4020 [PubMed] [Google Scholar]
- 6. Metcalf TU, Griffin DE. 2011. Alphavirus-induced encephalomyelitis: antibody-secreting cells and viral clearance from the nervous system. J. Virol. 85:11490–11501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Burgoon MP, Keays KM, Owens GP, Ritchie AM, Rai PR, Cool CD, Gilden DH. 2005. Laser-capture microdissection of plasma cells from subacute sclerosing panencephalitis brain reveals intrathecal disease-relevant antibodies. Proc. Natl. Acad. Sci. U. S. A. 102:7245–7250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Owens GP, Ritchie AM, Gilden DH, Burgoon MP, Becker D, Bennett JL. 2007. Measles virus-specific plasma cells are prominent in subacute sclerosing panencephalitis CSF. Neurology 68:1815–1819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Reuter D, Schneider-Schaulies J. 2010. Measles virus infection of the CNS: human disease, animal models, and approaches to therapy. Med. Microbiol. Immunol. 199:261–271 [DOI] [PubMed] [Google Scholar]
- 10. Stewart BS, Demarest VL, Wong SJ, Green S, Bernard KA. 2011. Persistence of virus-specific immune responses in the central nervous system of mice after West Nile virus infection. BMC Immunol. 12:6 doi:10.1186/1471-2172-12-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hooper DC, Phares TW, Fabis MJ, Roy A. 2009. The production of antibody by invading B cells is required for the clearance of rabies virus from the central nervous system. PLoS Negl. Trop. Dis. 3:e535 doi:10.1371/journal.pntd.0000535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mokhtarian F, Huan CM, Roman C, Raine CS. 2003. Semliki Forest virus-induced demyelination and remyelination—involvement of B cells and anti-myelin antibodies. J. Neuroimmunol. 137:19–31 [DOI] [PubMed] [Google Scholar]
- 13. Parsons LM, Webb HE. 1989. Identification of immunoglobulin-containing cells in the central nervous system of the mouse following infection with the demyelinating strain of Semliki Forest virus. Br. J. Exp. Pathol. 70:247–255 [PMC free article] [PubMed] [Google Scholar]
- 14. Pachner AR, Brady J, Narayan K. 2007. Antibody-secreting cells in the central nervous system in an animal model of MS: Phenotype, association with disability, and in vitro production of antibody. J. Neuroimmunol. 190:112–120 [DOI] [PubMed] [Google Scholar]
- 15. Tschen SI, Bergmann CC, Ramakrishna C, Morales S, Atkinson R, Stohlman SA. 2002. Recruitment kinetics and composition of antibody-secreting cells within the central nervous system following viral encephalomyelitis. J. Immunol. 168:2922–2929 [DOI] [PubMed] [Google Scholar]
- 16. Tschen SI, Stohlman SA, Ramakrishna C, Hinton DR, Atkinson RD, Bergmann CC. 2006. CNS viral infection diverts homing of antibody-secreting cells from lymphoid organs to the CNS. Eur. J. Immunol. 36:603–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lin MT, Hinton DR, Marten NW, Bergmann CC, Stohlman SA. 1999. Antibody prevents virus reactivation within the central nervous system. J. Immunol. 162:7358–7368 [PubMed] [Google Scholar]
- 18. Marques CP, Kapil P, Hinton DR, Hindinger C, Nutt SL, Ransohoff RM, Phares TW, Stohlman SA, Bergmann CC. 2011. CXCR3-dependent plasma blast migration to the central nervous system during viral encephalomyelitis. J. Virol. 85:6136–6147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Benson MJ, Dillon SR, Castigli E, Geha RS, Xu S, Lam KP, Noelle RJ. 2008. Cutting edge: the dependence of plasma cells and independence of memory B cells on BAFF and APRIL. J. Immunol. 180:3655–3659 [DOI] [PubMed] [Google Scholar]
- 20. Moser K, Tokoyoda K, Radbruch A, MacLennan I, Manz RA. 2006. Stromal niches, plasma cell differentiation and survival. Curr. Opin. Immunol. 18:265–270 [DOI] [PubMed] [Google Scholar]
- 21. Franciotta D, Salvetti M, Lolli F, Serafini B, Aloisi F. 2008. B cells and multiple sclerosis. Lancet Neurol. 7:852–858 [DOI] [PubMed] [Google Scholar]
- 22. Corcione A, Casazza S, Ferretti E, Giunti D, Zappia E, Pistorio A, Gambini C, Mancardi GL, Uccelli A, Pistoia V. 2004. Recapitulation of B cell differentiation in the central nervous system of patients with multiple sclerosis. Proc. Natl. Acad. Sci. U. S. A. 101:11064–11069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Krumbholz M, Theil D, Cepok S, Hemmer B, Kivisakk P, Ransohoff RM, Hofbauer M, Farina C, Derfuss T, Hartle C, Newcombe J, Hohlfeld R, Meinl E. 2006. Chemokines in multiple sclerosis: CXCL12 and CXCL13 up-regulation is differentially linked to CNS immune cell recruitment. Brain 129:200–211 [DOI] [PubMed] [Google Scholar]
- 24. Krumbholz M, Theil D, Derfuss T, Rosenwald A, Schrader F, Monoranu CM, Kalled SL, Hess DM, Serafini B, Aloisi F, Wekerle H, Hohlfeld R, Meinl E. 2005. BAFF is produced by astrocytes and up-regulated in multiple sclerosis lesions and primary central nervous system lymphoma. J. Exp. Med. 201:195–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Serafini B, Rosicarelli B, Magliozzi R, Stigliano E, Aloisi F. 2004. Detection of ectopic B-cell follicles with germinal centers in the meninges of patients with secondary progressive multiple sclerosis. Brain Pathol. 14:164–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Szczucinski A, Losy J. 2007. Chemokines and chemokine receptors in multiple sclerosis. Potential targets for new therapies. Acta Neurol. Scand. 115:137–146 [DOI] [PubMed] [Google Scholar]
- 27. Lustig S, Jackson AC, Hahn CS, Griffin DE, Strauss EG, Strauss JH. 1988. The molecular basis of Sindbis virus neurovirulence in mice. J. Virol. 62:2329–2336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Liu MT, Lane TE. 2001. Chemokine expression and viral infection of the central nervous system: regulation of host defense and neuropathology. Immunol. Res. 24:111–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Takeshita Y, Ransohoff RM. 2012. Inflammatory cell trafficking across the blood-brain barrier: chemokine regulation and in vitro models. Immunol. Rev. 248:228–239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Semple BD, Kossmann T, Morganti-Kossmann MC. 2010. Role of chemokines in CNS health and pathology: a focus on the CCL2/CCR2 and CXCL8/CXCR2 networks. J. Cereb. Blood Flow Metab. 30:459–473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lalor SJ, Segal BM. 2010. Lymphoid chemokines in the CNS. J. Neuroimmunol. 224:56–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Moll NM, Ransohoff RM. 2010. CXCL12 and CXCR4 in bone marrow physiology. Expert Rev. Hematol. 3:315–322 [DOI] [PubMed] [Google Scholar]
- 33. Scholzen T, Gerdes J. 2000. The Ki-67 protein: from the known and the unknown. J. Cell. Physiol. 182:311–322 [DOI] [PubMed] [Google Scholar]
- 34. Blink EJ, Light A, Kallies A, Nutt SL, Hodgkin PD, Tarlinton DM. 2005. Early appearance of germinal center-derived memory B cells and plasma cells in blood after primary immunization. J. Exp. Med. 201:545–554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. McHeyzer-Williams MG, Ahmed R. 1999. B cell memory and the long-lived plasma cell. Curr. Opin. Immunol. 11:172–179 [DOI] [PubMed] [Google Scholar]
- 36. Meinl E, Krumbholz M, Hohlfeld R. 2006. B lineage cells in the inflammatory central nervous system environment: migration, maintenance, local antibody production, and therapeutic modulation. Ann. Neurol. 59:880–892 [DOI] [PubMed] [Google Scholar]
- 37. Cassese G, Arce S, Hauser AE, Lehnert K, Moewes B, Mostarac M, Muehlinghaus G, Szyska M, Radbruch A, Manz RA. 2003. Plasma cell survival is mediated by synergistic effects of cytokines and adhesion-dependent signals. J. Immunol. 171:1684–1690 [DOI] [PubMed] [Google Scholar]
- 38. Klein B, Tarte K, Jourdan M, Mathouk K, Moreaux J, Jourdan E, Legouffe E, De VJ, Rossi JF. 2003. Survival and proliferation factors of normal and malignant plasma cells. Int. J. Hematol. 78:106–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Konforte D, Simard N, Paige CJ. 2009. IL-21: an executor of B cell fate. J. Immunol. 182:1781–1787 [DOI] [PubMed] [Google Scholar]
- 40. Zotos D, Tarlinton DM. 2012. Determining germinal centre B cell fate. Trends Immunol. 33:281–288 [DOI] [PubMed] [Google Scholar]
- 41. Chang SH, Dong C. 2011. Signaling of interleukin-17 family cytokines in immunity and inflammation. Cell Signal. 23:1069–1075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Mackay F, Schneider P. 2008. TACI, an enigmatic BAFF/APRIL receptor, with new unappreciated biochemical and biological properties. Cytokine Growth Factor Rev. 19:263–276 [DOI] [PubMed] [Google Scholar]
- 43. Thangarajh M, Masterman T, Hillert J, Moerk S, Jonsson R. 2007. A proliferation-inducing ligand (APRIL) is expressed by astrocytes and is increased in multiple sclerosis. Scand. J. Immunol. 65:92–98 [DOI] [PubMed] [Google Scholar]
- 44. Farina C, Aloisi F, Meinl E. 2007. Astrocytes are active players in cerebral innate immunity. Trends Immunol. 28:138–145 [DOI] [PubMed] [Google Scholar]
- 45. Patterson CE, Daley JK, Echols LA, Lane TE, Rall GF. 2003. Measles virus infection induces chemokine synthesis by neurons. J. Immunol. 171:3102–3109 [DOI] [PubMed] [Google Scholar]
- 46. Hauser AE, Debes GF, Arce S, Cassese G, Hamann A, Radbruch A, Manz RA. 2002. Chemotactic responsiveness toward ligands for CXCR3 and CXCR4 is regulated on plasma blasts during the time course of a memory immune response. J. Immunol. 169:1277–1282 [DOI] [PubMed] [Google Scholar]
- 47. Spolski R, Leonard WJ. 2010. IL-21 is an immune activator that also mediates suppression via IL-10. Crit. Rev. Immunol. 30:559–570 [DOI] [PubMed] [Google Scholar]
- 48. Phares TW, Marques CP, Stohlman SA, Hinton DR, Bergmann CC. 2011. Factors supporting intrathecal humoral responses following viral encephalomyelitis. J. Virol. 85:2589–2598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kim KS, Park JY, Jou I, Park SM. 2009. Functional implication of BAFF synthesis and release in gangliosides-stimulated microglia. J. Leukoc. Biol. 86:349–359 [DOI] [PubMed] [Google Scholar]
- 50. Uccelli A, Aloisi F, Pistoia V. 2005. Unveiling the enigma of the CNS as a B-cell fostering environment. Trends Immunol. 26:254–259 [DOI] [PubMed] [Google Scholar]
- 51. Rainey-Barger EK, Rumble JM, Lalor SJ, Esen N, Segal BM, Irani DN. 2011. The lymphoid chemokine, CXCL13, is dispensable for the initial recruitment of B cells to the acutely inflamed central nervous system. Brain Behav. Immun. 25:922–931 [DOI] [PMC free article] [PubMed] [Google Scholar]