Fig 4.
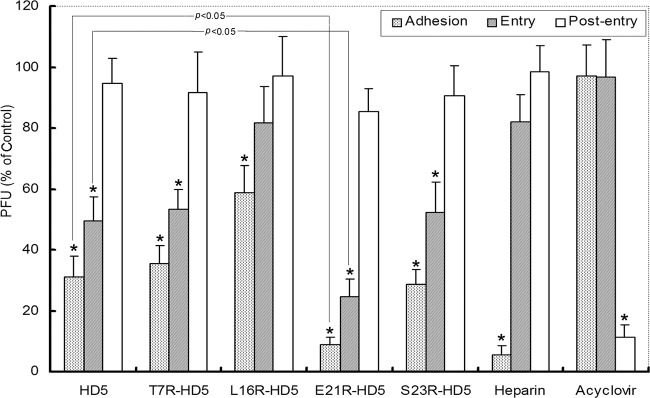
Synchronized infectivity assays demonstrated that HD5 analogs inhibited viral adhesion and entry to target cells in the viral life cycle. CaSki cells were cooled to 4°C and incubated with ∼200 PFU/well at 4°C for 3 h. After the unbound viruses were washed off, the cultures were shifted to 37°C for 30 min to admit viral entry. Adhered but nonpenetrant viruses were inactivated by washing the monolayer with a pH 3.0 buffer, after which the cells were overlaid with 2% methylcellulose-DMEM. Plaques were numerated 72 h postinfection. Peptides (50 μg/ml), heparin (100 μg/ml), acyclovir (100 μg/ml), or control buffer was added at the onset of each infection step. The bars display PFU formed in the presence of peptides as a percentage of PFU formed in the control wells and represent the means ± SD of three independent experiments. All five peptides significantly inhibited viral adhesion and/or entry (indicated by the asterisks) (P < 0.05). E21R-HD5 showed significantly enhanced antiviral efficacy compared to that of natural HD5 (P < 0.05). Heparin only blocked adhesion, and acyclovir inhibited infection only when added in the postentry stage.
