Abstract
Attenuated Semliki Forest virus (SFV) may be suitable for targeting malignant glioma due to its natural neurotropism, but its replication in brain tumor cells may be restricted by innate antiviral defenses. We attempted to facilitate SFV replication in glioma cells by combining it with vaccinia virus, which is capable of antagonizing such defenses. Surprisingly, we found parenchymal mouse brain tumors to be refractory to both viruses. Also, vaccinia virus appears to be sensitive to SFV-induced antiviral interference.
TEXT
Brain tumors are particularly life-threatening due to their sensitive anatomical location. Recently, temozolomide plus radiotherapy has provided a measurable survival benefit to a subset of patients (1), but more effective therapies are still needed. In this regard, oncolytic viruses (OVs) seem particularly promising, as they display higher tumor specificity and possibly fewer side effects than standard therapies (2). One of our OV candidates, attenuated Semliki Forest virus (SFV), was able to fully eradicate orthotopic U87 xenografts in 100% of treated nude mice following a single systemic injection (3). However, in other models, we and others have identified limitations to oncolytic virotherapy; in particular, innate antiviral defenses limit virus replication in tumor cells (4, 5).
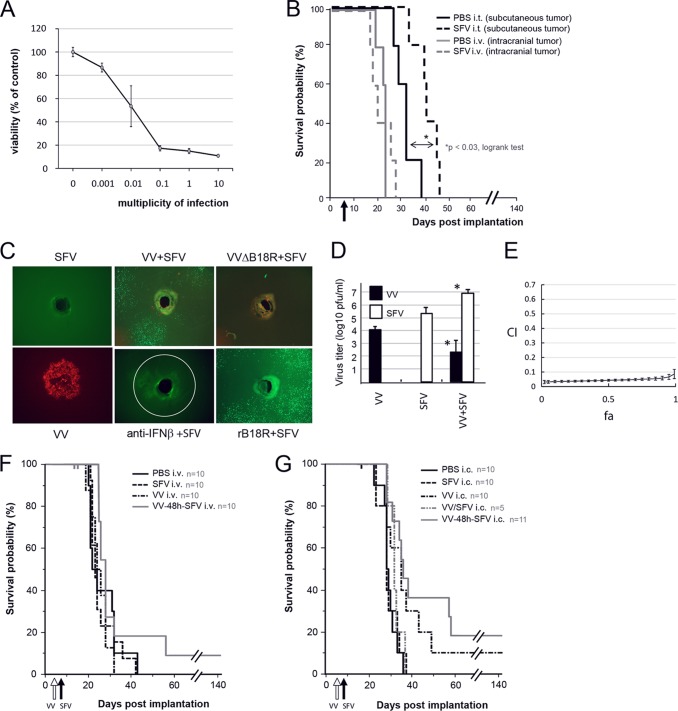
In order to probe further and overcome the mechanisms of glioma resistance to oncolytic SFV, we combined it with oncolytic vaccinia virus (VV), which itself has shown promise in glioma targeting and also has the capacity to facilitate replication of type I interferon (IFN)-sensitive OVs by antagonizing innate antiviral defenses (6–8). First, we demonstrate efficient killing of DBT mouse glioma cells in vitro but not in vivo by SFV alone, mirroring the results we observed in our previous rat model (4) (Fig. 1A and B). Lack of efficacy could not be explained simply by the immune competence of the animals, as SFV successfully eradicated another type of syngeneic tumor (CT26LacZ) at a similar dose (not shown). Next, we observed that SFV limits its own spread in DBT cells under spatially restrictive conditions (under agarose) and that this limitation could be lifted by coinfecting cells with VV or neutralizing type I IFN using polyclonal antibody (anti-beta interferon [IFN-β]) or recombinant vaccinia virus-encoded B18R protein (Fig. 1C). Facilitation of SFV spread in DBT cells by VV is dependent on B18R, as we did not see enhancement when B18R-deleted VV was used. While the spread of SFV under agarose was enhanced when SFV was combined with VV, replication of VV itself was strongly inhibited (Fig. 1C), which was also confirmed by quantifying virus output from coinfected DBT cells in free culture (Fig. 1D). DBT cell killing by the virus combinations was synergistic, as calculated by the Chou-Talalay method (Fig. 1E). However, combination of SFV with VV in an orthotopic DBT model did not provide statistically significant improvement in survival compared to the next best therapy, VV alone, by either systemic injection or direct intracranial administration or when viruses were coinjected or given 48 h apart (P < 0.0729, log rank test) (Fig. 1F and G). Moreover, systemic delivery of the viruses resulted in one mouse of five displaying hind leg paralysis in two separate experiments. Following intravenous administration, VV is known to reduce the ability of plasmacytoid dendritic cells to secrete type I interferon, thereby increasing systemic infection levels and pathogenesis of at least lymphocytic choriomeningitis virus and Pichinde virus (9, 10).
Fig 1.
Vaccinia virus facilitates replication of Semliki Forest virus in mouse glioma cells in culture but not in vivo. (A) Dose-dependent killing of BALB/c mouse DBT glioma cells by SFV in vitro, assessed by alamarBlue measurement from triplicate wells 48 h postinfection. (B) Apart from a transient delay in subcutaneous DBT tumor growth upon a single intratumoral injection of 108 PFU SFV, efficacy of SFV in the DBT model is lacking. (C) Plaque expansion assay (20) in DBT cell monolayers in 6-well plates showed that while SFV is unable to spread on its own under agarose, VV coinfection as well as VV-encoded soluble type I interferon scavenger protein B18R (0.1 μg per well; eBioscience, San Diego, CA) and neutralizing polyclonal IFN-β antibody (peripheral blood lymphocyte interferon source) can overcome this restriction, confirming that type I IFN may limit SFV replication in DBT cells. (D) Plaque assay of infected DBT cell culture supernatants (VV MOI, 0.1; SFV MOI, 0.01) shows that while SFV replication is enhanced in DBT cells when SFV is combined with VV at 48 h p.i., replication of VV is significantly inhibited in the presence of SFV (*, P < 0.02 [Mann-Whitney U test]). (E) SFV and VV synergize in cell killing, as measured 72 h postinfection in DBT cells infected with various ratios of VV to SFV. The plot represents the algebraic estimate of the combination index (CI) as a function of the fraction of cells affected (fa) ± the standard deviation, where a CI of <0.7 is considered synergistic. (F) A single systemic injection of 108 PFU of VV followed 48 h later by SFV into mice harboring intracranial (day 6 postimplantation) DBT tumors showed a trend toward increased survival compared to the next treatment, VV alone (P < 0.0729 [log rank test]). One mouse in each of two experiments in the combination group displayed hind leg paralysis and was promptly sacrificed (censored). (G) Survival of mice treated intracranially with 107 PFU virus (sum of two experiments). While the results for the sequential (VV–48-h–SFV) combination were statistically different from those for the VV-SFV-coinjected group and the group receiving SFV alone (P < 0.0132 [log rank test]), they did not differ statistically from those for the group receiving VV alone, which in turn did not differ from those for any other group. Censored animals are shown as small vertical bars along the curves (F and G, top).
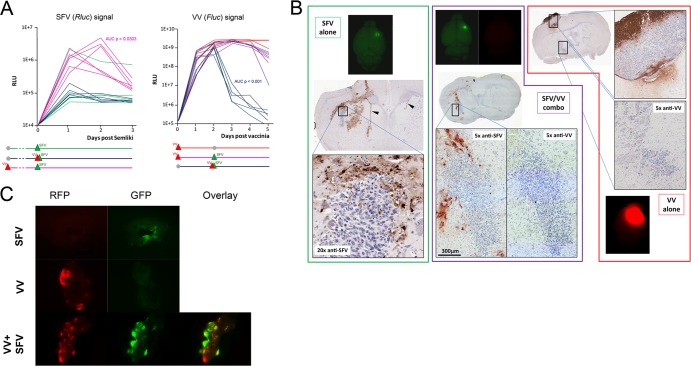
In order to understand whether the difference in treatment efficacy between coinjected and 48-h-sequenced treatment groups (VV-SFV versus VV–48-h–SFV, P < 0.0037, log rank test) (Fig. 1G) was due to inhibition of VV replication by SFV or due to enhancement of SFV by VV, we tracked virus replication in the brains of tumor-bearing animals using luciferase-encoding viruses. Correlating with survival data and arguing for both mechanisms, replication of intracranially injected SFV was enhanced by VV but only when VV was given 48 h before SFV, and, conversely, VV replication was dramatically inhibited only when VV was coinjected with SFV (Fig. 2A). However, since heterologous virus interference did not occur when VV was given 48 h prior to SFV, we still needed to find an explanation for the overall lack of therapeutic efficacy of the combination in the intracranial DBT model. Subsequently, upon immunohistochemical examination of the brains of tumor-bearing virus-treated mice using virus-specific polyclonal rabbit antibodies, we observed a lack of infection of intraparenchymal DBT tumors by either virus (Fig. 2B). This demonstrated that when growing in the brain, DBT cells acquire resistance to both type I IFN-sensitive and IFN-insensitive viruses, suggesting broad-spectrum IFN-independent mechanisms of antiviral resistance. Our results also suggest that virus-expressed luminescence (Fig. 2A) originates from infected normal cells in the case of SFV and from tumor tissue growing outside the brain parenchyma in the case of VV (Fig. 2B).
Fig 2.
Mouse glioma growing in the brain is not permissive to oncolytic SFV or VV. (A) Day 6 DBT tumor-bearing mice were injected intracranially with Renilla luciferase-expressing SFV, with firefly luciferase-expressing VV, or with both viruses given either in the same injection or 48 h apart; virus replication was tracked by the IVIS in vivo imaging system over 5 days (72 h after the last administration of virus). Results show transient (up to 2 days) enhancement of SFV signal in mice that were sequentially treated (48 h apart) but not in those that were coinjected. Conversely, VV replication is strongly inhibited by simultaneous coadministration of SFV but not when SFV is given 48 h after VV. (B) Immunohistochemical analysis of brains sampled 24 h postinfection shows absence of infection of DBT tumor tissue deep in the brain by either SFV or VV or their comixture but prominent infection of healthy brain by SFV. VV is able to infect only DBT tumor tissue growing outside the brain parenchyma. (C) In contrast to the results obtained in vivo, SFV is able to infect DBT brain tumor explants and VV is able to enhance SFV replication in them. Tumor pieces were infected with 107 PFU of each virus immediately upon extraction from the brain and visualized 24 h postinfection.
As DBT cells are permissive to both SFV and VV in vitro, we wanted to explore the stability of the brain-dependent virus resistance; therefore, we extracted DBT tumor tissue from the brains of tumor-bearing mice and infected it ex vivo. We observed infection of explanted tumor tissue by each virus alone as well as facilitation of SFV replication when SFV was combined with VV (Fig. 2C), mirroring our findings in cultured DBT cells (Fig. 1C and D) and corroborating the idea that DBT cells acquire reversible and context-dependent antiviral resistance when implanted and growing in the brain. In line with our findings, a recent collaborative report showed that GL261 mouse glioma cells preinfected with SFV at very high MOI in vitro (and thus destined to succumb to the infection) formed intracranial tumors with an efficiency similar to that of uninfected glioma cells (5).
Both tumor stroma (11) and the brain microenvironment (12) significantly modulate global gene expression pathways in glioma cells, making them more resistant to a variety of challenges in vivo than in vitro. Such mechanisms may not only hinder oncolytic SFV or VV but also underlie the previously reported reduced replication of oncolytic adenovirus in intracranial gliomas compared to cultured glioma cells (13). While reducing type I IFN-mediated antiviral defenses has been shown to enhance replication and oncolytic efficacy of several other OVs in other tumor models (14–17), our paper underscores the fact that such strategies may not automatically translate into better therapy and may be hazardous if not controlled precisely. Fortunately, glioma resistance to OVs may be context dependent and reversible, which opens the possibility of developing specific countermeasures. Also, combination therapy of cancer with heterologous viruses, even with SFV and VV, is still an appealing concept, as safety may be increased by using replication-defective vectors or vectors carrying microRNA targets or by employing prime-boost-type vaccination regimens where virus injections are temporally separated and do not allow uncontrolled virus enhancement (18, 19).
ACKNOWLEDGMENTS
DBT cells were a kind gift from Robert C. Rostomily, University of Washington, School of Medicine, Seattle, WA.
This project was funded by the Academy of Finland (grant 125186), Canadian Institutes of Health Research, and Terry Fox Foundation, Canada. F. Le Boeuf was supported by an Industrial Fellowship from the Canadian Institutes for Health Research. A. Hinkkanen was financially supported by the Cancer Centre of Eastern Finland, Kuopio University Hospital, and the Academy of Finland.
John Bell is cofounder of Jennerex Biotherapeutics and sits on the board of directors. Akseli Hemminki is cofounder and shareholder of Oncos Therapeutics.
Footnotes
Published ahead of print 5 December 2012
REFERENCES
- 1. Stupp R, Hegi M, Weller M. 2010. Neuro-oncology, a decade of temozolomide and beyond. Expert. Rev. Anticancer Ther. 10:1675–1677 [DOI] [PubMed] [Google Scholar]
- 2. Guo ZS, Thorne SH, Bartlett DL. 2008. Oncolytic virotherapy: molecular targets in tumor-selective replication and carrier cell-mediated delivery of oncolytic viruses. Biochim. Biophys. Acta 1785:217–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Heikkilä JE, Vähä-Koskela MJ, Ruotsalainen JJ, Martikainen MW, Stanford MM, McCart JA, Bell JC, Hinkkanen AE. 2010. Intravenously administered alphavirus vector VA7 eradicates orthotopic human glioma xenografts in nude mice. PLoS One 5:e8603 doi:10.1371/journal.pone.0008603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mättä AM, Liimatainen T, Wahlfors T, Wirth T, Vähä-Koskela M, Jansson L, Valonen P, Hakkinen K, Rautsi O, Pellinen R, Mäkinen K, Hakumäki J, Hinkkanen A, Wahlfors J. 2007. Evaluation of cancer virotherapy with attenuated replicative Semliki forest virus in different rodent tumor models. Int. J. Cancer 121:863–870 [DOI] [PubMed] [Google Scholar]
- 5. Ruotsalainen J, Martikainen M, Niittykoski M, Huhtala T, Aaltonen T, Heikkilä J, Bell J, Vähä-Koskela M, Hinkkanen A. 2012. Interferon-beta sensitivity of tumor cells correlates with poor response to VA7 virotherapy in mouse glioma models. Mol. Ther. 20:1529–1539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Le Boeuf F, Diallo JS, McCart JA, Thorne S, Falls T, Stanford M, Kanji F, Auer R, Brown CW, Lichty BD, Parato K, Atkins H, Kirn D, Bell JC. 2010. Synergistic interaction between oncolytic viruses augments tumor killing. Mol. Ther. 18:888–895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Le Boeuf F, Bell JC. 2010. United virus: the oncolytic tag-team against cancer! Cytokine Growth Factor Rev. 21:205–211 [DOI] [PubMed] [Google Scholar]
- 8. Lun XQ, Jang JH, Tang N, Deng H, Head R, Bell JC, Stojdl DF, Nutt CL, Senger DL, Forsyth PA, McCart JA. 2009. Efficacy of systemically administered oncolytic vaccinia virotherapy for malignant gliomas is enhanced by combination therapy with rapamycin or cyclophosphamide. Clin. Cancer Res. 15:2777–2788 [DOI] [PubMed] [Google Scholar]
- 9. Welsh RM, Che JW, Brehm MA, Selin LK. 2010. Heterologous immunity between viruses. Immunol. Rev. 235:244–266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zuniga EI, Liou LY, Mack L, Mendoza M, Oldstone MB. 2008. Persistent virus infection inhibits type I interferon production by plasmacytoid dendritic cells to facilitate opportunistic infections. Cell Host Microbe 4:374–386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. McMillin DW, Delmore J, Weisberg E, Negri JM, Geer DC, Klippel S, Mitsiades N, Schlossman RL, Munshi NC, Kung AL, Griffin JD, Richardson PG, Anderson KC, Mitsiades CS. 2010. Tumor cell-specific bioluminescence platform to identify stroma-induced changes to anticancer drug activity. Nat. Med. 16:483–489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Camphausen K, Purow B, Sproull M, Scott T, Ozawa T, Deen DF, Tofilon PJ. 2005. Influence of in vivo growth on human glioma cell line gene expression: convergent profiles under orthotopic conditions. Proc. Natl. Acad. Sci. U. S. A. 102:8287–8292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lamfers ML, Idema S, Bosscher L, Heukelom S, Moeniralm S, van der Meulen-Muileman IH, Overmeer RM, van d van Beusechem VVW, Gerritsen WR, Vandertop WP, Dirven CM. 2007. Differential effects of combined Ad5- delta 24RGD and radiation therapy in in vitro versus in vivo models of malignant glioma. Clin. Cancer Res. 13:7451–7458 [DOI] [PubMed] [Google Scholar]
- 14. Huang PY, Guo JH, Hwang LH. 2012. Oncolytic Sindbis virus targets tumors defective in the interferon response and induces significant bystander antitumor immunity in vivo. Mol. Ther. 20:298–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Krishnamurthy S, Takimoto T, Scroggs RA, Portner A. 2006. Differentially regulated interferon response determines the outcome of Newcastle disease virus infection in normal and tumor cell lines. J. Virol. 80:5145–5155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Naik S, Russell SJ. 2009. Engineering oncolytic viruses to exploit tumor specific defects in innate immune signaling pathways. Expert. Opin. Biol. Ther. 9:1163–1176 [DOI] [PubMed] [Google Scholar]
- 17. Paglino JC, van den Pol AN. 2011. Vesicular stomatitis virus has extensive oncolytic activity against human sarcomas: rare resistance is overcome by blocking interferon pathways. J. Virol. 85:9346–9358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Näslund TI, Uyttenhove C, Nordström EK, Colau D, Warnier G, Jondal M, Van den Eynde BJ, Liljeström P. 2007. Comparative prime-boost vaccinations using Semliki Forest virus, adenovirus, and ALVAC vectors demonstrate differences in the generation of a protective central memory CTL response against the P815 tumor. J. Immunol. 178:6761–6769 [DOI] [PubMed] [Google Scholar]
- 19. Zhang YQ, Tsai YC, Monie A, Wu TC, Hung CF. 2010. Enhancing the therapeutic effect against ovarian cancer through a combination of viral oncolysis and antigen-specific immunotherapy. Mol. Ther. 18:692–699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Duca KA, Lam V, Keren I, Endler EE, Letchworth GJ, Novella IS, Yin J. 2001. Quantifying viral propagation in vitro: toward a method for characterization of complex phenotypes. Biotechnol. Prog. 17:1156–1165 [DOI] [PubMed] [Google Scholar]