Abstract
Background
Attainment of remission is viewed as the optimal outcome of acute antidepressant treatment. However, some patients experience subsyndromal symptoms after they achieve remission. This study examines the prognostic significance of subsyndromal symptoms occurring during the first six months after remission of late-life depression.
Methods
Older (60–89 years) inpatients and outpatients, with unipolar major depression, were followed until remission (asymptomatic or almost asymptomatic for 3 consecutive weeks). Two hundred and forty-two achieved remission after uncontrolled antidepressant treatment. This analysis focused on remitted patients who had follow-up data over a 2.5 year period (N=185).
Results
Approximately 18% of patients relapsed. Of the remainder (n=152), 42.8% had subsyndromal depressive symptoms during the 6 months following remission. Cox’s Proportional Survival Analysis demonstrated that longer duration of subsyndromal symptoms (number of weeks with LIFE-Psychiatric Status Rating Scale (PSR) score of 3 or 4) in the first 6 months after remission was significantly associated with shorter time to recurrence and higher recurrence rate (Hazard Ratio:1.16; 95% CI= 1.08–1.24). Based on our analysis, patients with 0, 4, 8, and 12 weeks of subsyndromal symptoms in the first six months after remission have estimated recurrence rates of 28%, 45%, 66%, or 86%, respectively, during the ensuing two years.
Conclusions
These findings highlight the clinical importance of subsyndromal symptoms occurring after remission in late-life depression. They also argue that studies of geriatric depression may complement the definition of remission with information on subsyndromal symptoms occurring after the initial asymptomatic period.
INTRODUCTION
Depression is a relapsing and recurring illness. Beyond suffering and compromised quality of life (Charney et al. 2003; Kessler et al. 2006), prolonged depression in late life increases medical burden and mortality both in medically ill and medically healthy persons (Bruce et al. 1994; Steffens et al. 1994). Disability conferred by late-life depression increases dependency needs, care costs, family disruption and often leads to institutionalization (Alexopolous et al. 2002).
Attainment of remission is viewed as the optimal outcome of acute antidepressant treatment (Anderson et al. 2008; Nierenberg & Wright 1999; Thase & Ninan 2002) in both young and older adults. Remission, defined as an almost asymptomatic state, is considered a stable clinical state with a low risk for relapse and recurrence (Fava et al. 2007; Frank et al. 1991; Rush et al. 2006). Patients who improve, but fail to achieve remission, have significant functional impairment, compromised quality of life, and high utilization of health care services (Miller et al. 1998; Simon et al. 2006). While remission is desirable, remission rates in mixed-age samples of patients with major depression treated with first-line antidepressant treatment are 30–35% and decline significantly with successive treatment failures (Nelson 2006). Remission may even be more difficult to achieve and sustain in depressed older patients (Mueller et al. 2004; Reynolds et al. 1996).
Several definitions of remission have been proposed. Most definitions specify two conditions. Attainment of an almost asymptomatic state over a predetermined period of time, usually 1–2 weeks (Frank et al. 1991; Rush et al. 2006). Inherent in these definitions is the assumption that once remission is achieved, patients would continue to have favorable long-term outcomes (Rush et al. 2006; Paykel et al. 1995; Thase & Simons 1992). Empirical studies support this point of view in both young adults and late-life depression since depressed patients who achieve remission have a lower relapse rate than those who improve but remain symptomatic (Rush et al. 2006; Ramana et al. 1995, Van Londen et al. 1998). However, patients who remain asymptomatic for the short period of time required to satisfy the definition of remission may still experience subsyndromal symptoms after remission.
Achievement of remission is often followed by development of subsyndromal depressive symptoms affecting up to 50% of patients (Nelson 2006; Rush 2007). In some cases, subsyndromal depressive symptoms are self-limited, but in others they may evolve into a recurrence of depression. This study focuses on the prognostic significance of subsyndromal depressive symptoms during the six months following remission of late-life depression, the period during which if a full blown depressive episode occurs it would be classified as a relapse (Nierenberg & Wright 1999; Riso et al. 1997). It is hypothesized that long duration of subsyndromal symptoms during the six months following attainment of remission is a predictor of recurrence.
METHODS
Subjects
The subjects were outpatients and inpatients of a psychiatric university hospital consecutively recruited for an uncontrolled treatment study of geriatric depression. There were no subjects recruited through advertisement. Inclusion criteria were: 1) age of 60 years or older, 2) diagnosis of unipolar Major Depressive Disorder according to DSM-IV; 3) a Longitudinal Follow-up Examination (LIFE) Psychiatric Status Rating Scale (PSR) score >=5 (5=Definite Criteria for MDD/Without Prominent Psychotic Symptoms or Extreme Impairment) (Keller et al. 1987); (3) the presence of an informant who has knowledge of the patient’s history and had enough contact so that he or she could observe behavioral changes within 1 week; and (4) residence at a distance within 45-minute from the psychiatric university hospital. All participants received uncontrolled treatment for depression. After complete description of the study, subjects signed written informed consent approved by the Weill-Cornell Medical College Institutional Review Board.
Exclusion criteria were: 1) a history of psychiatric illness (except personality disorder) other than unipolar depressive disorder diagnosed prior to the onset of the first depressive episode; (2) severe acute medical illness, i.e. metastatic cancer, decompensated cardiac, liver of renal failure, major surgery, and stroke or myocardial infarction 6 months prior to study entry; 3) spinocerebellar degeneration, Huntington’s chorea, Parkinson’s disease, or multi-infarct dementia; 4) moderate to severe dementia as evidenced by a Mini-Mental State Examination (Folstein et al. 1975) MMSE score lower than 20; and 5) severe behavioral disability defined as complete impairment in performance of activities of daily living domain of the Philadelphia Multilevel Assessment Instrument (MAI) (Lawton et al. 1982). These criteria resulted in a group of older patients with unipolar major depression but without a major medical illness, moderate or severe dementia, or severe disability.
Systematic Assessment
At baseline, diagnostic assessment was based on the SCID-R. Depression severity was assessed with the Psychiatric Status Rating Scale (PSR) of Longitudinal Interval Follow-up Evaluation (LIFE) (Keller et al. 1987). Cognitive impairment was measured with the Mini-Mental State Examination (MMSE) (Folstein et al. 1975) and Mattis Dementia Rating Scale (DRS) (Mattis 1989). Medical burden was quantified using the total score (except the psychiatric domain) of the Cumulative Illness Rating Scale, Modified Version for Geriatrics (CIRS-G) (Miller et al. 1992). CIRS-G Information was obtained from the medical history and physical examination, as well as the available laboratory test results. Disability was assessed with the Instrumental Activities of Daily Living (IADL) subscale of the Philadelphia Multilevel Assessment Instrument (MAI); the IADL subscale provides information about the participant’s ability to perform nine IADLs (Lawton et. al 1982).
After the baseline evaluation, the patients and informants (when necessary) had in-person evaluations every 6 months and telephone evaluations 3 months in between each in-person session. An extra in-person evaluation was conducted when the participant first met criteria for remission and for recurrence. If the participants were unable to come to the hospital, research assistants visited them at their homes. During follow-up evaluations, depressive symptoms were assessed with the PSR. The PSR assessed the presence and severity of depressive symptoms each week during the 6 months prior to each assessment. Memory prompts and detailed questioning are used to improve the accuracy of information. When patients are unable to offer information and when discrepancies are detected, raters interview individuals who have frequent contact with the patients and/or contact the patients’ own physicians to obtain additional information. Thus the PSR utilizes an information gathering process similar to that of a clinical examination. The PSR classifies the status of depression on a scale of 1 to 6. PSR score 1 refers to an “Asymptomatic Status/Returned to Usual Self” status without any residual symptoms of the MDD disorder. PSR score 2 refers to “Residual/Mild Depressive Symptoms” with presence of one or more symptoms of no more than the mild degree. PSR score 3 refers to “Subsyndromal Symptoms/Moderate Symptoms” with no more than moderate impairment in functioning. PSR score 4 refers to “Major Symptoms” with major impairment in functioning but does not meet full criteria of MDD. PSR score 5 refers to “Definite Criteria for MDD/Without Prominent Psychotic Symptoms or Extreme Impairment”. Finally, PSR score=6 refers to “Definite Criteria for MDD/With Psychotic Symptoms or Extreme Impairment” (Keller et al. 1987; Judd et al. 1998). Cognitive impairment (MMSE), medical burden (CIRS-G), and disability (IADL Domain of MAI) were assessed at two time points, at baseline, when the MDD diagnosis was made and when they first met criteria for remission.
Intensity of antidepressant drug treatment (AD) was classified with the Composite Antidepressant Score – Revised (CAD) (Alexopoulos et al. 1996). CAD orders intensity of AD treatment on a scale of 0–4 with 0=no medication; 1 corresponds to treatment with daily doses of Buproprion <100 mg, Nortriptyline <30, Setraline <25 mg, Paroxetine, Fluoxetine, or Citalopram <10 mg, Venlafaxine <50 mg, or Escitalopram < 5 mg; 2 corresponds to Buproprion 100–199 mg, Nortriptyline 30–49, Setraline 25–49 mg, Paroxetine, Fluoxetine, or Citalopram 10–19 mg, Venlafaxine 50–75 mg, or Escitalopram 5–10 mg; 3 corresponds to Buproprion 200–300 mg, Nortriptyline 50–80 mg, Sertraline 50–100 mg, Paroxetine, Fluoxetine, or Citalopram 20–30 mg, Venlafaxine 76–150 mg, or Escitalopram 10–15 mg; and 4 corresponds to Buproprion >300 mg, Nortriptyline >80 mg, Setraline >100 mg, Paroxetine, Fluoxetine, or Citalopram >30 mg, Venlafaxine >150 mg, or Escitalopram >15 mg. The investigators had no control over treatment with antidepressants. The antidepressants were prescribed by community-based physicians responsible for the patients’ clinical care.
Definitions of Remission, Duration of Subsyndromal Symptoms, and Recurrence
In this study, remission was defined as a PSR score of <=2 for 3 consecutive weeks, without the presence of depressed mood or lack of interest or pleasure, after an episode of MDD. This definition is consistent a) with studies of late-life depression (Alexopoulos et al. 2009; Bhalla et al. 2006; Rutherford et al. 2007; Taylor et al. 2011), in which the required duration for remission has been 1–3 consecutive weeks of a specific cut-off score; and b) with the recommendations from the ACNP Task Force on Remission and Response (Fava, Ruini & Belaise 2007). Relapse was defined as a PSR score>=5 during the first six months after remission. The duration of subsyndromal symptoms after remission (“Duration Subsyndromal Symptoms”) was calculated by the number of weeks of PSR scores of 3 or 4 in the first 6 months following remission in subjects who did not meet criteria for relapse. Recurrence was defined as a PSR score >=5 occurring between 6 months to 2½ years (last research observation since baseline) after remission.
Statistical Analysis
Bivariate analyses compared subjects who had a recurrence of depression with subjects who remained well using Chi-square and Wilcoxon-Mann-Whitney tests. Then, Cox Proportional Hazards Survival Analysess were performed to explore the association of “Duration Subsyndromal Symptoms” during the risk period for relapse (in the first six months following remission) with time to recurrence. Any participant who did not have a recurrence was considered censored. To examine whether the effect of “Duration Subsyndromal Symptoms” on time to recurrence remained significant in the presence of confounding variables, we created a final Cox Proportional Hazards regression model including variables that were associated with time to recurrence (Using Cox Proportional Hazards models and p<0.15). The hazard rates of each predictor were tested for proportionality with a Cox model including the variable and its interaction with log(time to recurrence) – 5.69. The constant, 5.69, is the average of the logs of the survival times. For the final Cox regression model, we used backward elimination with p=0.05 as the criterion for removing an explanatory variable from the model. All analyses were performed with SAS 9.1 and S-Plus 7.0.
RESULTS
A total of 317 older outpatients (N=191) and inpatients (N=126) who met DSM-IV criteria for Major Depressive Disorder entered this study. Of these, 242 achieved remission after receiving uncontrolled antidepressant treatment, 42 did not remit and 33 were lost to follow-up or had incomplete data to determine remission. There were no significant differences between those who were lost to follow-up or had incomplete data (N=33) vs. the rest (N=284) on age, entry status (inpatient vs. outpatient), and years of education. However, patients who were lost to follow-up or had incomplete data had a significantly higher percentage of males than the rest 284 patients (Males percentage: 15.45% vs 7.22%, chi-square=5.47, df=1, p=0.019).
Among the subjects who achieved remission (N=242), 57 were lost to follow-up or had incomplete data in the first 6 months after remission. This report focuses on the 185 subjects who remained in remission for 6 months and had follow-up data for up to 2.5 years thereafter. The subjects who were lost to follow-up or had incomplete data were older by an average of 4.37 years than the 185 subjects with follow-up data (Mann-Whitney: z=3.08, p=0.0021). Further, a higher percentage of inpatients were lost to follow-up or had incomplete data compared to subjects with follow-up (50.88% vs. 31.35%, chi-square=7.22, df=1, p=0.0072). There were no significant differences on gender and years of education between the two groups.
Relapse and Subsyndromal Symptoms
Despite the stringent definition of remission requiring 3 weeks of an almost asymptomatic state, 17.8% (33/185) of remitted subjects experienced a relapse during the 6 months following remission. Subjects who relapsed were older and more cognitively impaired at entry than those who remained relapse-free (Table 1).
Table 1.
Demographic and Clinical Characteristics on 185 Older Subjects with Major Depression Followed for 6 Months after Remission.
| Had a Relapse | Remained Relapse-free | |||||||
|---|---|---|---|---|---|---|---|---|
| (N=33) | (N=152) | |||||||
| N | Perc (%) | N | Perc (%) | Chi-square | p | |||
| Gender | 0.0004 | 0.98 | ||||||
| Female | 21 | 63.64% | 97 | 63.82% | ||||
| Entry Status at Last MDD Episode | 2.29 | 0.13 | ||||||
| Inpatient | 14 | 42.42% | 44 | 28.95% | ||||
| Outpatient | 19 | 57.58% | 108 | 71.05% | ||||
| Mann-Whitney Wilcoxon | ||||||||
| Mean | SD | Range | Mean | SD | Range | z | p | |
| Age | 75.12 | 6.27 | 64–86 | 71.96 | 7 | 60–89 | 2.41 | 0.02 |
| Education (years) | 13.18 | 3.88 | 5–21 | 14.17 | 3.09 | 7–21 | −1.62 | 0.1 |
| Age at Onset | 59.34 | 16.65 | 30–83 | 56.45 | 18.27 | 14–87 | 0.81 | 0.42 |
| Duration of Last Episode | 53.25 | 42.46 | 3–174 | 66.5 | 74.75 | 3–522 | −0.26 | 0.79 |
| Number MDD Episodes | 2.45 | 1.41 | 1–6 | 2.05 | 1.24 | 1–7 | 1.6 | 0.11 |
| Entry (Index Episode) | ||||||||
| DRS Total1 | 133.74 | 7.11 | 116–144 | 136.47 | 7.23 | 111–144 | −2.07 | 0.0387 |
| CIRS Total2 | 6.23 | 3.13 | 2–3 | 5.51 | 3.24 | 0–19 | 1.08 | 0.28 |
| IADL3 | 24 | 3.73 | 15–27 | 25.56 | 2.32 | 15–27 | −1.66 | 0.1 |
| At Onset of Remission4 | ||||||||
| DRS Total1 | 130.54 | 12.93 | 100–143 | 137.34 | 6.8 | 103–144 | −1.88 | 0.0593 |
| CIRS Total2 | 5.96 | 3.23 | 0–12 | 4.6 | 3.14 | 0–13 | 1.93 | 0.06 |
| IADL3 | 25.2 | 3.29 | 17–27 | 25.77 | 2.28 | 15–27 | −0.12 | 0.91 |
| Antidepressant (AD) Drug Treatment | ||||||||
| Intensity5 | 2.42 | 1.47 | 0–4 | 2.17 | 1.61 | 0–4 | 0.57 | 0.57 |
Mattis Dementia Rating Scale;
Cumulative Illness Rating Scale-Geriatric Version;
Philadelphia Multilevel Assessment Instrument – IADL Subscale;
When remission was first identified
Composite Antidepressant Score (CAD) in last month before relapse or in the sixth month.
Even among participants who remained relapse free within 6 months after remission (N=152), a rather high number developed clinically significant depressive symptomatology. Specifically, only 57.24% (87/152) were asymptomatic or had mild residual depressive symptoms (PSR scores of 1 and 2) during the 6-month period following remission, while 42.76% (65/152) of relapse-free subjects developed subsyndromal symptoms (PSR scores of 3 or 4). In this last group (N=65), 47.69% (31/65) subsyndromal symptoms lasted up to 4 weeks, 38.46% (25/65) lasted 4–8 weeks, and 13.85% (9/65) lasted longer than 8 weeks.
Recurrence
Further analysis focused on the 152 depressed elderly subjects who achieved remission and remained relapse free for 6 months. Of the 152 subjects, 108 were outpatients and 44 were inpatients. Most depressed subjects had a recurrent disorder (Table 2). Their average age at onset was in midlife. On average, their index episode was of moderate severity. They had significant medical burden, mild or no cognitive impairment, and some impairment in instrumental activities of daily living.
Table 2.
Demographic and Clinical Characteristics on 152 Older Subjects with Major Depression Who Remained in Remission Past the Risk Period for Relapse and Were Followed for Two Years.
| Had a Recurrence | Remained Recurrence-free | |||||||
|---|---|---|---|---|---|---|---|---|
| (N=45) | (N=152) | |||||||
| N | Perc (%) | N | Perc (%) | Chi-square | p | |||
| Gender | 2.51 | 0.11 | ||||||
| Female | 33 | 73.33% | 64 | 59.81% | ||||
| Entry Status at Last MDD Episode | 2.49 | 0.12 | ||||||
| Inpatient | 9 | 20.00% | 35 | 32.71% | ||||
| Outpatient | 36 | 80.00% | 72 | 67.29% | ||||
| Mann-Whitney Wilcoxon | ||||||||
| Mean | SD | Range | Mean | SD | Range | z | p | |
| Age | 72.27 | 6.73 | 60–89 | 71.83 | 7.14 | 60–89 | 0.31 | 0.76 |
| Education (years) | 14.00 | 3.10 | 8–20 | 14.24 | 3.10 | 7–21 | −0.31 | 0.75 |
| Duration of Subsyndromal Symptoms | 3.64 | 3.82 | 0–14 | 1.33 | 2.66 | 0–14 | 4.00 | <0.0001 |
| Age at Onset | 58.34 | 17.46 | 20–87 | 55.65 | 18.64 | 14–83 | 0.74 | 0.46 |
| Duration of Last Episode | 89.62 | 106.40 | 11–522 | 57.67 | 54.51 | 3–283 | 1.49 | 0.14 |
| Number MDD Episodes | 1.94 | 1.08 | 1–6 | 2.09 | 1.31 | 1–7 | −0.30 | 0.76 |
| At Entry (Index Episode) | ||||||||
| DRS Total1 | 137.60 | 5.52 | 127–144 | 135.98 | 7.85 | 111–144 | 0.66 | 0.51 |
| CIRS Total2 | 5.68 | 2.74 | 1–12 | 5.45 | 3.42 | 0–19 | 0.83 | 0.41 |
| IADL3 | 25.53 | 1.80 | 20–27 | 25.57 | 2.50 | 15–27 | −1.24 | 0.21 |
| At Onset of Remission4 | ||||||||
| DRS Total1 | 138.00 | 5.58 | 119–144 | 137.06 | 7.28 | 103–144 | 0.48 | 0.63 |
| CIRS Total2 | 4.29 | 3.12 | 0–12 | 4.73 | 3.16 | 0–13 | −0.73 | 0.46 |
| IADL3 | 26.21 | 1.03 | 23–27 | 25.63 | 2.55 | 15–27 | −0.34 | 0.74 |
| Antidepressant (AD) Medication Treatment | ||||||||
| Intensity of AD Treatment5 | 2.44 | 1.39 | 0–4 | 2.53 | 1.41 | 0–4 | −0.53 | 0.6 |
Mattis Dementia Rating Scale;
Cumulative Illness Rating Scale-Geriatric Version;
Philadelphia Multilevel Assessment Instrument – IADL;
When remission was first identified
Composite Antidepressant Score (CAD) in last month before recurrence or before last follow-up.
Of the 152 subjects who remained in remission of depression past the relapse risk period (6 months), 45 had a recurrence (a PSR score >= 5) during the ensuing 2 years (2.5 years since remission). After taking into consideration censored participants, the estimated recurrence rate derived from the Cox Proportional Hazards model during the 2 years of follow-up was 37.6% (95% CI: 29.2% – 47.6%). Bivariate comparisons between subjects who suffered a recurrence and those who remained well showed no significant difference in any demographic or clinical variables. The only exception was that subjects who suffered a recurrence had a longer duration of subsyndromal symptoms in the six months following remission of depression than subjects who remained well throughout the follow-up period (Table 2).
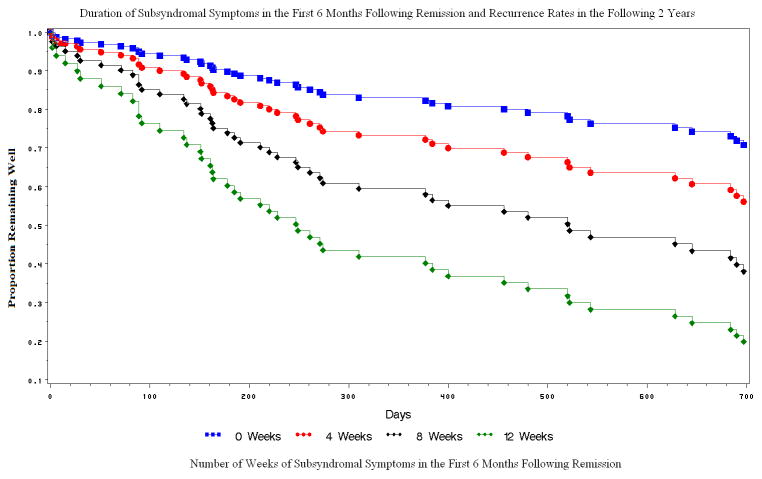
The duration of subsyndromal symptoms was significantly associated with time to recurrence (Wald Chi-square=18.28, p<0.0001, Hazard Ratio=1.16, 95% Hazard Ratio Confidence Interval=1.08 – 1.24); the higher the number of weekly PSR scores of 3 or 4 in the first 6 months after remission, the shorter the time to recurrence. Specifically, participants with 4, 8, and 12 weeks of PSR score of 3 or 4 in the first 6 months after remission had 45%, 66%, and 86% recurrence rates respectively (Figure 1).
Figure 1. Duration of Subsyndromal Symptoms in the First 6 Months Following Remission and Recurrence Rates in the Ensuing 2 Years.
Survival curves of time to recurrence of elderly depressed patients who experience 0, 4, 8, and 12 weeks of subsyndromal symptoms during the first 6 months following remission. The survival curves start 6 months after remission (0 days) and end 2 years later. Calculated by the Proportional Hazards Model: “Duration Subsyndromal Symptoms” associated with time to recurrence. Proportional Hazards Likelihood Ratio for the overall model: χ2==13.74, df=1, p<0.0002; for Duration Subsyndromal Symptoms: χ2=18.28, df=1, p=<.0001.
Further analysis examined whether the relationship of subsyndromal symptom duration to recurrence remained significant in the presence of potential confounding variables. To this end, exploratory univariate analyses were conducted using Cox Proportional Hazards Model to identify demographic and clinical variables related to time to recurrence with a p value equal to or lower than 0.15 (Table 3). These variables were included in a multivariate Cox Proportional Hazards Model.
Table 3.
Bivariate Relationships of Demographic and Clinical Variables to Time to Recurrence in 152 Older Subjects with Major Depression Who Remained in Remission Past the Risk Period for Relapse.
| Variables | Cox Proportional Hazards Model | ||
|---|---|---|---|
| Hazard Ratio (95% CI) | Wald χ2 | p | |
| Age | 1.01 (0.97–1.06) | 0.22 | 0.64 |
| Education | 0.99 (0.90–1.09) | 0.04 | 0.83 |
| Gender | 1.60 (0.83–3.11) | 1.97 | 0.16 |
| Outpatient/Inpatient Status at Entry | 1.41 (0.95–2.08) | 2.91 | 0.08 |
| Duration Subsyndromal Symptoms | 1.16 (1.08–1.24) | 18.28 | <.0001 |
| Duration of Index MDD Episode | 1.00 (1.00–1.06) | 2.85 | 0.09 |
| Age at Onset of MDD | 1.00 (0.99–1.02) | 0.57 | 0.45 |
| Number MDD Episodes | 0.97 (0.94–1.29) | 0.16 | 0.69 |
| At Entry | |||
| DRS Total1 | 1.03 (0.99–1.08) | 1.80 | 0.18 |
| CIRS Total2 | 1.07 (0.97–1.18) | 1.77 | 0.18 |
| IADL3 | 0.95 (0.84–1.10) | 0.39 | 0.53 |
| At Onset of Remission | |||
| DRS Total1 | 1.02 (0.97–1.07) | 0.51 | 0.48 |
| CIRS Total2 | 0.98 (0.88–1.09) | 0.15 | 0.70 |
| IADL3 | 1.07 (0.89–1.29) | 0.44 | 0.51 |
| Intensity of AD Treatment4 | 1.12 (0.85–1.47) | 0.62 | 0.43 |
| Intensity of AD Treat X Time§ | 1.23 (1.03–1.46) | 5.41 | 0.02 |
Time=log(time to recurrence)-5.69; The constant, 5.69, is the average of the logs of the survival times. The hazard ratio of Intensity of AD Treatment scores was not proportional over time as shown by the significant Intensity of AD Treat x Time interaction
Mattis Dementia Rating Scale
Cumulative Illness Rating Scale-Geriatric Version
Philadelphia Multilevel Assessment Instrument – IADL Subscale
Composite Antidepressant Score (CAD) in last month before recurrence or before last follow-up.
The final Cox predictive model of recurrence was constructed after use of backward elimination in which variables with p>0.05 were removed. The resultant model consisted of “Duration Subsyndromal Symptoms” (Wald Chi-square=18.72, p<0.0001, Hazard Ratio=1.17, 95% Hazard Ratio Confidence Interval=1.09 – 1.25), Intensity of AD treatment (Wald Chi-square=0.64, p=0.42, Hazard Ratio=1.12, 95% Hazard Ratio Confidence Interval=0.85 – 1.48), and Intensity of AD treatment x Time (Wald Chi-square=5.78, p=0.016, Hazard Ratio=1.24, 95% Hazard Ratio Confidence Interval=1.04 – 1.49).
To further assure that our continuous measure of intensity of antidepressant treatment (CAD scores ranging from 0–4) adequately captured the role of treatment, we used a categorical definition of antidepressant treatment (adequate vs. inadequate) and repeated the above analysis. According to this definition, as “adequate” was characterized treatment with CAD intensity ≥3 and “inadequate” was treatment with a CAD intensity ≤. In a Cox’s proportional hazard model including Adequate Antidepressant Treatment, Duration of Subsyndromal Symptoms, and the interaction of Adequate Antidepressant Treatment x Time, the results were similar to the previous final model. Specifically, “Duration of Subsyndromal Symptoms” (Wald Chi-square=18.69, p<0.0001, Hazard Ratio=1.17, 95% Hazard Ratio Confidence Interval=1.09 – 1.25), Adequacy of AD treatment (Wald Chi-square=0.51, p=0.47, Hazard Ratio=1.35, 95% Hazard Ratio Confidence Interval=0.60 – 3.05), and Adequacy of AD treatment x Time (Wald Chi-square=6.01, p=0.014, Hazard Ratio=2.23, 95% Hazard Ratio Confidence Interval=1.17 – 4.23).
The significant interaction of adequacy of antidepressant treatment with time indicates that the relationship of treatment to recurrence was not the same over time. Specifically, during the first 4 months of follow-up, patients with adequate antidepressant treatment were 5 times less likely to recur than patients with inadequate antidepressant treatment (Wald Chi-square=7.82, p=0.0052, HR=5.37, 95% Hazard Ratio Confidence Interval =1.65–17.46). However, adequacy of antidepressant treatment after the first 4 months of follow-up was not significantly associated with reduced recurrence rates (i.e. after 10 months following remission) (Wald Chi-square=1.50, p=0.23, HR=0.52; 95% Hazard Ratio Confidence Interval =0.23 – 1.40).
DISCUSSION
The principal finding of this study is that subsyndromal symptoms in the first 6 months after remission are common and predict subsequent recurrence of late-life depression; the longer the duration of subsyndromal symptoms, the shorter the time to recurrence and the higher the recurrence rates. Older patients who experience subsyndromal symptoms for 0, 4, 8, and 12 weeks during the six months after remission have an estimated recurrence rate of 28%, 45%, 66% and 86% respectively during the ensuing two years. The relationship of subsyndromal symptoms to recurrence is independent of the intensity of antidepressant drug treatment during the month preceding recurrence.
To our knowledge, this is the first study to demonstrate that increased duration of subsyndromal symptoms occurring soon (six months) after remission is associated with increased risk for recurrence of late-life depression. Nonetheless, this observation is consistent with findings from controlled and uncontrolled treatment studies suggesting that subsyndromal symptoms forecast poor long-term outcomes in elderly and in younger patients (Paykel et al. 1995; Thase & Simons 1992; Ramana et al. 1995; Judd et al. 1998; Van Londen et al. 1998; Dombrovski et al. 2007; Frank & Levenson 1990; Karp et al. 2004; Taylor et al. 1999). With one exception (Frank & Levenson 1990), studies of adults documented that subsyndromal symptoms at the point of remission or during the continuation and maintenance treatment phases were associated with high recurrence rates during the next two years (Ramana et al. 1995; Judd et al. 1998; Karp et al. 2004). In late life, subsyndromal depressive symptoms occurring prior to or during the maintenance treatment phase predicted subsequent recurrence of major depression (Dombrovski et al. 2007; Taylor et al. 1999). Finally, recent evidence suggests that treating subsyndromal depression symptoms may prevent major depression in late life (van’t Veer-Tazelaar et al. 2009).
This study’s findings need to be viewed in the context of this study’s limitations. These include the lack of controlled treatment and the absence of a control group. While uncontrolled treatment may be a limitation, the treatment offered to the subjects of this study was of low to medium intensity (Table 1 and 2). While a control group is desirable, the lack of a randomization reduced the subject selection bias inherent in randomized treatment studies. Another limitation may be the retrospective assessment of depressive symptoms at six-month intervals and a telephone assessment in between. While direct assessment of depressive symptoms at more frequent intervals is desirable, the LIFE and PSR employed by this study are instruments validated by the NIMH Collaborative Study of Depression (Keller et al. 1987), and have been used in studies with mixed-age (Judd et al. 1998, Keller et al. 1992) and elderly depressed patients (Hinrichsen 1992). Finally, the MMSE cut-off of 20 may have allowed inclusion of individuals with cognitive impairment or early stage dementia. A wide range of cognitive impairment coexists with depression in older adults and increases with age (Arve 1999). A more stringent MMSE cut-off score would have resulted in a sample of younger cognitively intact, and relatively healthy participants and not accurately capture important clinical aspects of an older population differentiating them from younger adults.
A number of clinical characteristics have been associated with poor outcomes of late-life depression. Overall cognitive dysfunction (Story et al. 2008), impairment in some executive functions (Alexopoulos et al. 2005; Sneed et al. 2007), and psychomotor slowing (Butters et al. 2004) each has been associated with slow and/or poor response to antidepressant treatment. Similarly, medical burden has been shown to predict poor long-term outcomes of late life depression in some studies (Cui et al. 2008). Finally, disability has a reciprocal relationship with depressive symptoms and worsens the long-term prognosis of late-life depression (Alexopoulos et al. 2002). In this study, however, cognitive impairment, medical burden, and disability assessed both at entry, when the subjects were symptomatic, and at the beginning of remission (Table 3) had weak associations with recurrence of depression that did not reach statistical significance. However, cognitive impairment and medical burden were associated with relapse in the first 6 months after remission (Table 1). Consequently, the group of the recurrence analyses was enriched with patients with low cognitive impairment and medical burden since many of those with greater cognitive impairment and medical burden had already relapsed. Nevertheless, further investigation of the association of medical comorbidity and cognitive impairment with recurrence rates is warranted.
The adequacy of antidepressant drug treatment during the first four months of follow-up predicted low recurrence rates. However, later on, adequacy of drug treatment did not significantly influence recurrence rates. Since a high number of recurrences occurred during the first 4 months of follow-up, the group with longer follow-up might have been depleted from patients at high risk for recurrence. This observation may account, at least in part, for the lack of a significant relationship between treatment adequacy after the first four months of follow-up and recurrence of depression.
The findings of this study have methodological and clinical implications. On a methodological level, long-term outcome studies of geriatric depression may include information on subsyndromal states occurring soon after the attainment of remission because of their prognostic value. In this study, focusing only on remission defined as of an almost asymptomatic state over three weeks predicted an average recurrence rate of 37.6% over two years. However, the occurrence and duration of subsyndromal depressive symptoms following remission increased the prediction of recurrence. For example, a patient without any subsyndromal symptoms after remission had a 28% chance of recurrence within two years. In contrast, the probability of recurrence of a patient with subsyndromal depressive symptoms lasting 12 weeks was 86%.
On a clinical level, while remission is a desirable outcome, it should not be viewed as a permanent state and attention shall be given to subsyndromal depressive symptoms developing after remission. Vigilant follow-up should aim to identify depressive symptoms after remission of late-life depression and treatment plans should be formed aiming to shorten their duration. While suppressing subsyndromal symptoms may reduce suffering and disability, studies are needed to establish whether it reduces recurrence rates. The possibility remains that subsyndromal symptoms are an index of a “virulent” disorder whose outcomes may change little with symptom suppression.
In conclusion, this study documents that a large percentage of depressed older patients develop subsyndromal depressive symptoms after remission of major depression. The duration of subsyndromal symptoms after remission is proportionately associated with recurrence of depression in the ensuing two years. While the mechanisms relating subsyndromal symptoms to recurrence are unclear it is prudent to remain clinically vigilant during the first few months after remission of late life depression.
Acknowledgments
NIH Grant Support: This work was supported by NIMH grants R01 MH042819 (Longitudinal Study of Late-Life Depression; PI: Alexopoulos), P30 MH085943 (Cornell ACISR in Late-Life Depression; PI: Alexopoulos), K23 MH074659 (Treating Depressed, Cognitively Impaired Elders; PI: Kiosses) and R01 MH091045 (Home-delivered Intervention for Depressed, Cognitively Impaired Elders; PI: Kiosses).
Dr. Kiosses received grant support from National Alliance for Research on Depression and Schizophrenia (NARSAD), the Mental Health Initiative Foundation, and Alzheimer’s Association. Dr. Alexopoulos received grant support from Forest; served as a consultant to Lilly; and has been a member of speakers and bureaus sponsored by Astra Zeneca, Forest, Merck, Avanir, and Lundbeck and a stockholder of Johnson and Johnson.
Footnotes
Disclosures: Dr. Alexopoulos had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. The conflicts of interests and financial disclosures are complete for Dr. Kiosses and Dr. Alexopoulos, to the best of their knowledge.
Contributor Information
Dimitris N. Kiosses, Weill-Cornell Institute of Geriatric Psychiatry, Weill Medical College of Cornell University.
George S. Alexopoulos, Weill-Cornell Institute of Geriatric Psychiatry, Weill Medical College of Cornell University.
References
- Alexopoulos GS, Buckwalter K, Olin J, Martinez R, Wainscott C, Krishnan KR. Comorbidity of late life depression: an opportunity for research on mechanisms and treatment. Biological Psychiatry. 2002;52:543–558. doi: 10.1016/s0006-3223(02)01468-3. [DOI] [PubMed] [Google Scholar]
- Alexopoulos GS, Kiosses DN, Heo M, Murphy CF, Shanmugham B, Gunning-Dixon F. Executive dysfunction and the course of geriatric depression. Biological Psychiatry. 2005;58:204–210. doi: 10.1016/j.biopsych.2005.04.024. [DOI] [PubMed] [Google Scholar]
- Alexopoulos GS, Meyers BS, Young RC, Kakuma T, Feder M, Einhorn A, Rosendahl E. Recovery in geriatric depression. Archives of General Psychiatry. 1996;53:305–312. doi: 10.1001/archpsyc.1996.01830040039008. [DOI] [PubMed] [Google Scholar]
- Alexopoulos GS, Reynolds CF, 3rd, Bruce ML, Katz IR, Raue PJ, Mulsant BH, Oslin DW, Ten Have T PROSPECT Group . Reducing suicidal ideation and depression in older primary care patients: 24-month outcomes of the PROSPECT study. American Journal of Psychiatry. 2009;166:882–890. doi: 10.1176/appi.ajp.2009.08121779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson IM, Ferrier IN, Baldwin RC, Cowen PJ, Howard L, Lewis G, Matthews K, McAllister-Williams RH, Peveler RC, Scott J, Tylee A. Evidence-based guidelines for treating depressive disorders with antidepressants: a revision of the 2000 British Association for Psychopharmacology guidelines. Journal of Psychopharmacology. 2008;22:343–396. doi: 10.1177/0269881107088441. [DOI] [PubMed] [Google Scholar]
- Arve S, Tilvis RS, Lehtonen A, Valvanne J, Sairanen S. Coexistence of lowered mood and cognitive impairment of elderly people in five birth cohorts. Aging (Milano) 1999;11:90–95. [PubMed] [Google Scholar]
- Bhalla RK, Butters MA, Mulsant BH, Begley AE, Zmuda MD, Schoderbek B, Pollock BG, Reynolds CF, 3rd, Becker JT. Persistence of neuropsychologic deficits in the remitted state of late-life depression. American Journal of Geriatric Psychiatry. 2006;14:419–427. doi: 10.1097/01.JGP.0000203130.45421.69. [DOI] [PubMed] [Google Scholar]
- Bruce ML, Leaf PJ, Rozal GP, Florio L, Hoff RA. Psychiatric status and 9-year mortality data in the New Haven Epidemiologic Catchment Area Study. American Journal of Psychiatry. 1994;151:716–721. doi: 10.1176/ajp.151.5.716. [DOI] [PubMed] [Google Scholar]
- Butters MA, Whyte EM, Nebes RD, Begley AE, Dew MA, Mulsant BH, Zmuda MD, Bhalla R, Meltzer CC, Pollock BG, Reynolds CF, 3rd, Becker JT. The nature and determinants of neuropsychological functioning in late-life depression. Archives of General Psychiatry. 2004;61:587–595. doi: 10.1001/archpsyc.61.6.587. [DOI] [PubMed] [Google Scholar]
- Charney DS, Reynolds CF, 3rd, Lewis L, Lebowitz BD, Sunderland T, Alexopoulos GS, Blazer DG, Katz IR, Meyers BS, Arean PA, Borson S, Brown C, Bruce ML, Callahan CM, Charlson ME, Conwell Y, Cuthbert BN, Devanand DP, Gibson MJ, Gottlieb GL, Krishnan KR, Laden SK, Lyketsos CG, Mulsant BH, Niederehe G, Olin JT, Oslin DW, Pearson J, Persky T, Pollock BG, Raetzman S, Reynolds M, Salzman C, Schulz R, Schwenk TL, Scolnick E, Unutzer J, Weissman MM, Young RC. Depression and Bipolar Support Alliance consensus statement on the unmet needs in diagnosis and treatment of mood disorders in late life. Archives of General Psychiatry. 2003;60:664–672. doi: 10.1001/archpsyc.60.7.664. [DOI] [PubMed] [Google Scholar]
- Cui X, Lyness JM, Tang W, Tu X, Conwell Y. Outcomes and predictors of late-life depression trajectories in older primary care patients. American Journal of Geriatric Psychiatry. 2008;16:406–415. doi: 10.1097/JGP.0b013e3181693264. [DOI] [PubMed] [Google Scholar]
- Dombrovski AY, Mulsant BH, Houck PR, Mazumdar S, Lenze EJ, Andreescu C, Cyranowski JM, Reynolds CF., 3rd Residual symptoms and recurrence during maintenance treatment of late-life depression. Journal of Affective Disorders. 2007;103:77–82. doi: 10.1016/j.jad.2007.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fava GA, Ruini C, Belaise C. The concept of recovery in major depression. Psychological Medicine. 2007;37:307–317. doi: 10.1017/S0033291706008981. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatry Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Frank EKD, Levenson J. Continuation therapy for unipolar depression: the case for combined treatment. In: Manning DM, editor. Combined Pharmacotherapy and Psychotherapy for Depression. Washington, DC: American Psychiatric Press; 1990. pp. 135–149. [Google Scholar]
- Frank E, Prien RF, Jarrett RB, Keller MB, Kupfer DJ, Lavori PW, Rush AJ, Weissman MM. Conceptualization and rationale for consensus definitions of terms in major depressive disorder. Remission, recovery, relapse, and recurrence. Archives of General Psychiatry. 1991;48:851–855. doi: 10.1001/archpsyc.1991.01810330075011. [DOI] [PubMed] [Google Scholar]
- Hinrichsen GA. Recovery and relapse from major depressive disorder in the elderly. American Journal of Psychiatry. 1992;149:1575–1579. doi: 10.1176/ajp.149.11.1575. [DOI] [PubMed] [Google Scholar]
- Judd LL, Akiskal HS, Maser JD, Zeller PJ, Endicott J, Coryell W, Paulus MP, Kunovac JL, Leon AC, Mueller TI, Rice JA, Keller MB. Major depressive disorder: a prospective study of residual subthreshold depressive symptoms as predictor of rapid relapse. Journal of Affective Disorders. 1998;50:97–108. doi: 10.1016/s0165-0327(98)00138-4. [DOI] [PubMed] [Google Scholar]
- Karp JF, Buysse DJ, Houck PR, Cherry C, Kupfer DJ, Frank E. Relationship of variability in residual symptoms with recurrence of major depressive disorder during maintenance treatment. American Journal of Psychiatry. 2004;161:1877–1884. doi: 10.1176/ajp.161.10.1877. [DOI] [PubMed] [Google Scholar]
- Keller MB, Lavori PW, Friedman B, Nielsen E, Endicott J, McDonald-Scott P, Andreasen NC. The Longitudinal Interval Follow-up Evaluation. A comprehensive method for assessing outcome in prospective longitudinal studies. Archives of General Psychiatry. 1987;44:540–548. doi: 10.1001/archpsyc.1987.01800180050009. [DOI] [PubMed] [Google Scholar]
- Keller MB, Lavori PW, Mueller TI, Endicott J, Coryell W, Hirschfeld RM, Shea T. Time to recovery, chronicity, and levels of psychopathology in major depression. A 5-year prospective follow-up of 431 subjects. Archives of General Psychiatry. 1992;49:809–816. doi: 10.1001/archpsyc.1992.01820100053010. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Akiskal HS, Ames M, Birnbaum H, Greenberg P, Hirschfeld RM, Jin R, Merikangas KR, Simon GE, Wang PS. Prevalence and effects of mood disorders on work performance in a nationally representative sample of U.S. workers. American Journal of Psychiatry. 2006;163:1561–1568. doi: 10.1176/appi.ajp.163.9.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawton MP, Moss M, Fulcomer M, Kleban MH. A research and service oriented multilevel assessment instrument. Journal of Gerontology. 1982;37:91–99. doi: 10.1093/geronj/37.1.91. [DOI] [PubMed] [Google Scholar]
- Mattis S. Dementia Rating Scale. Odessa, FL: Psychological Assessment Resources; 1989. [Google Scholar]
- Miller IW, Keitner GI, Schatzberg AF, Klein DN, Thase ME, Rush AJ, Markowitz JC, Schlager DS, Kornstein SG, Davis SM, Harrison WM, Keller MB. The treatment of chronic depression, part 3: psychosocial functioning before and after treatment with sertraline or imipramine. Journal of Clinical Psychiatry. 2008;59:608–619. doi: 10.4088/jcp.v59n1108. [DOI] [PubMed] [Google Scholar]
- Miller MD, Paradis CF, Houck PR, Mazumdar S, Stack JA, Rifai AH, Mulsant B, Reynolds CF., 3rd Rating chronic medical illness burden in geropsychiatric practice and research: application of the Cumulative Illness Rating Scale. Psychiatry Research. 1992;41:237–248. doi: 10.1016/0165-1781(92)90005-n. [DOI] [PubMed] [Google Scholar]
- Mueller TI, Kohn R, Leventhal N, Leon AC, Solomon D, Coryell W, Endicott J, Alexopoulos GS, Keller MB. The course of depression in elderly patients. American Journal of Geriatric Psychiatry. 2004;12:22–29. [PubMed] [Google Scholar]
- Nelson JC. The STAR*D study: a four-course meal that leaves us wanting more. American Journal of Psychiatry. 2006;163:1864–1866. doi: 10.1176/ajp.2006.163.11.1864. [DOI] [PubMed] [Google Scholar]
- Nierenberg AA, Wright EC. Evolution of remission as the new standard in the treatment of depression. Journal of Clinical Psychiatry. 1999;60(Suppl 22):7–11. [PubMed] [Google Scholar]
- Paykel ES, Ramana R, Cooper Z, Hayhurst H, Kerr J, Barocka A. Residual symptoms after partial remission: an important outcome in depression. Psychological Medicine. 1995;25:1171–1180. doi: 10.1017/s0033291700033146. [DOI] [PubMed] [Google Scholar]
- Ramana R, Paykel ES, Cooper Z, Hayhurst H, Saxty M, Surtees PG. Remission and relapse in major depression: a two-year prospective follow-up study. Psychological Medicine. 1995;25:1161–1170. doi: 10.1017/s0033291700033134. [DOI] [PubMed] [Google Scholar]
- Reynolds CF, 3rd, Frank E, Kupfer DJ, Thase ME, Perel JM, Mazumdar S, Houck PR. Treatment outcome in recurrent major depression: a post hoc comparison of elderly (“young old”) and midlife patients. American Journal of Psychiatry. 1996;153:1288–1292. doi: 10.1176/ajp.153.10.1288. [DOI] [PubMed] [Google Scholar]
- Riso LP, Thase ME, Howland RH, Friedman ES, Simons AD, Tu XM. A prospective test of criteria for response, remission, relapse, recovery, and recurrence in depressed patients treated with cognitive behavior therapy. Journal of Affective Disorders. 1997;43:131–142. doi: 10.1016/s0165-0327(96)01420-6. [DOI] [PubMed] [Google Scholar]
- Rush AJ. STAR*D: what have we learned? American Journal of Psychiatry. 2007;164:201–204. doi: 10.1176/ajp.2007.164.2.201. [DOI] [PubMed] [Google Scholar]
- Rush AJ, Kraemer HC, Sackeim HA, Fava M, Trivedi MH, Frank E, Ninan PT, Thase ME, Gelenberg AJ, Kupfer DJ, Regier DA, Rosenbaum JF, Ray O, Schatzberg AF ANCP Task Force. Report by the ACNP Task Force on response and remission in major depressive disorder. Neuropsychopharmacology. 2006;31:1841–1853. doi: 10.1038/sj.npp.1301131. [DOI] [PubMed] [Google Scholar]
- Rutherford B, Sneed J, Miyazaki M, Eisenstadt R, Devanand D, Sackeim H, Roose S. An open trial of aripiprazole augmentation for SSRI non-remitters with late-life depression. International Journal of Geriatric Psychiatry. 2007;22:986–991. doi: 10.1002/gps.1775. [DOI] [PubMed] [Google Scholar]
- Simon GE, Khandker RK, Ichikawa L, Operskalski BH. Recovery from depression predicts lower health services costs. Journal of Clinical Psychiatry. 2006;67:1226–1231. doi: 10.4088/jcp.v67n0808. [DOI] [PubMed] [Google Scholar]
- Sneed JR, Roose SP, Keilp JG, Krishnan KR, Alexopoulos GS, Sackeim HA. Response inhibition predicts poor antidepressant treatment response in very old depressed patients. American Journal of Geriatric Psychiatry. 2007;15:553–563. doi: 10.1097/JGP.0b013e3180302513. [DOI] [PubMed] [Google Scholar]
- Steffens DC, Levy RM, Wagner R, McQuoid DR, Krishnan KR, Carroll BJ. Sociodemographic and clinical predictors of mortality in geriatric depression. American Journal of Geriatric Psychiatry. 2002;10:531–540. [PubMed] [Google Scholar]
- Story TJ, Potter GG, Attix DK, Welsh-Bohmer KA, Steffens DC. Neurocognitive correlates of response to treatment in late-life depression. American Journal of Geriatric Psychiatry. 2008;16:752–759. doi: 10.1097/JGP.0b013e31817e739a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor WD, McQuoid DR, Ashley-Koch A, MacFall JR, Bridgers J, Krishnan RR, Steffens DC. BDNF Val66Met genotype and 6-month remission rates in late-life depression. Pharmacogenomics Journal. 2011;11:146–154. doi: 10.1038/tpj.2010.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor MP, Reynolds CF, 3rd, Frank E, Cornes C, Miller MD, Stack JA, Begley AE, Mazumdar S, Dew MA, Kupfer DJ. Which elderly depressed patients remain well on maintenance interpersonal psychotherapy alone?: report from the Pittsburg study of maintenance therapies in late-life depression. Depression and Anxiety. 1999;10:55–60. [PubMed] [Google Scholar]
- Thase ME, Ninan PT. New goals in the treatment of depression: moving toward recovery. Psychopharmacology Bulletin. 2002;36:24–35. [PubMed] [Google Scholar]
- Thase ME, Simons AD. Cognitive behavior therapy and relapse of nonbipolar depression: parallels with pharmacotherapy. Psychopharmacology Bulletin. 1992;28:117–122. [PubMed] [Google Scholar]
- Van Londen L, Molenaar RP, Goekoop JG, Zwinderman AH, Rooijmans HG. Three- to 5-year prospective follow-up of outcome in major depression. Psychological Medicine. 1998;28:731–735. doi: 10.1017/s0033291797006466. [DOI] [PubMed] [Google Scholar]
- van’t Veer-Tazelaar PJ, van Marwijk HW, van Oppen P, van Hout HP, van der Horst HE, Cuijpers P, Smit F, Beekman AT. Stepped-care prevention of anxiety and depression in late life: a randomized controlled trial. Arch Gen Psychiatry. 2009;66:297–304. doi: 10.1001/archgenpsychiatry.2008.555. [DOI] [PubMed] [Google Scholar]