This report identifies both climatological and air pollution constituents as independent risk factors for hospitalization of HIV-positive patients with Pneumocystis pneumonia (PcP). These findings may lead to new insights about the epidemiology and pathogenesis of PcP.
Keywords: Pneumocystis, PcP, environmental factors, HIV
Abstract
Background. Pneumocystis pneumonia (PcP) is the second leading cause of morbidity and mortality in human immunodeficiency virus (HIV)–infected patients in the United States. Although the host risk factors for the development of PcP are well established, the environmental (climatological, air pollution) risk factors are poorly understood. The major goal of this study was to determine the environmental risk factors for admissions of HIV-positive patients with PcP to a single medical center.
Methods. Between 1997 and 2008, 457 HIV-positive patients with microscopically confirmed PcP were admitted to the San Francisco General Hospital. A case-crossover design was applied to identify environmental risk factors for PcP hospitalizations. Climatological and air pollution data were collected from the Environmental Protection Agency and Weather Warehouse databases. Conditional logistic regression was used to evaluate the association of each environmental factor and PcP hospital admission.
Results. Hospital admissions were significantly more common in the summer than in the other seasons. Increases in temperature and sulfur dioxide levels were independently associated with hospital admissions for PcP, but the effects of sulfur dioxide were modified by increasing carbon monoxide levels.
Conclusions. This study identifies both climatological and air pollution constituents as independent risk factors for hospitalization of HIV-positive patients with PcP in San Francisco. Thus, the environmental effects on PcP are more likely complex than previously thought. Further studies are needed to understand how these factors exert their effects and to determine if these factors are associated with PcP in other geographic locations.
(See the Editorial Commentary by Hughes, on pages 82–3.)
Pneumocystis is an opportunistic fungal pathogen causing pneumonia (PcP) in human immunodeficiency virus (HIV)–infected and other immunocompromised patients [1, 2]. While the incidence of PcP has declined with the use of chemoprophylaxis and antiretroviral therapy, PcP remains the second leading cause of morbidity and mortality in HIV-positive patients [1]. Furthermore, Pneumocystis colonization of the respiratory tract has been found in HIV-positive and non-HIV-positive patients and to be associated with the development of chronic obstructive lung disease (COPD) [3, 4]. The events that lead from Pneumocystis colonization or mild infection to active PcP that requires hospitalization likely involve the interplay of host, organism, and environmental factors. The host factors (eg, CD4 cell count) are well established, and recent evidence suggests that Pneumocystis genotypes or virulence factors may also be involved [5].
In contrast, the role of specific environmental (climatological and air pollution) factors in the development of PcP are poorly understood. PcP in HIV-positive patients has been associated with outdoor activities (gardening, camping, hiking), and geographic clusters of PcP have occurred [6–8]. While there have been studies of the effects of seasonality on Pneumocystis colonization [9, 10], PcP incidence [11–16], and PcP outcome [17, 18], the results have been inconsistent.
Increased levels of air pollutants, including carbon monoxide (CO), nitrogen dioxide (NO2), ozone, sulfur dioxide (SO2), and particulate matter up to 10 μm in size (PM10) and up to 2.5 μm in size (PM2.5), are well-known risk factors for impaired lung function, pneumonia, asthma, COPD, and other pulmonary diseases [19–25]. Yet, the effects of air pollution on a patient's risk of developing and presenting with PcP are unknown. Potentially, high levels of air pollution might affect airway inflammation, reduce macrophage function, and exacerbate existing or evolving symptoms and thus result in patients seeking medical care.
The main goal of the present study was to determine the climatological and air pollution constituents that are independent risk factors for admission of HIV-positive patients with PcP to the San Francisco General Hospital (SFGH).
MATERIALS AND METHODS
Study Design
A case crossover design (CCD) was used to assess the environmental risk factors for “first-episode” PcP admissions of HIV-positive patients to SFGH. CCD has been used in numerous epidemiologic studies that have examined the association between air pollution and the likelihood of hospital admissions due to a variety of respiratory diseases [26, 28]. CCD is a type of matched case-control analysis in which a subject serves as his/her own control [27, 29]. For each environmental exposure factor examined in the present study, the significance of the relative effects of exposure levels at the same subject's previously determined “case” and “control” times were tested using conditional logistic regression to assess the association between that exposure and the likelihood of admission to SFGH with PcP.
Study Population
Consecutive HIV-positive patients admitted to SFGH between 1 January 1997 and 31 December 2008 and diagnosed with microscopically confirmed PcP (by demonstration of the trophic and/or cystic form of Pneumocystis jirovecii in bronchoalveolar lavage fluid or induced sputum using Diff Quik, a modified Giemsa stain) and who had previously been evaluated using a standard diagnostic protocol were studied [30]. Confirmation of PcP status and collection of demographic and clinical data were performed by review of medical and laboratory microbiology records as previously reported [31].
Timing of Measurements of Exposure Levels
Among HIV-positive persons, the time course from (environmental) exposure to Pneumocystis and development of clinically apparent PcP is not known. However, it is generally accepted that the period from the beginning of symptoms to the time of hospital presentation in these patents is about 4–8 weeks [32]. In light of this, we chose to compare the environmental exposure of individual patients at the time of presentation with PcP with 3 time periods: 2 months, 1 month, and 2 weeks before presentation to SFGH with PcP. For each time period studied, we looked at an average value for each environmental parameter, derived by averaging the values obtained over a 3-day period for each environmental factor being considered. This was done in order to eliminate an “extreme” level of that environmental factor, present on one day, which might have skewed the data and biased our interpretation. Three-day average exposure levels to temperature, humidity, CO, NO2, ozone, SO2, and PM10 (on the day of PcP diagnosis and the 2 days immediately prior to diagnosis), were analyzed to define exposure at the time of PcP hospital admission (case identification). For control periods, the exposures for the 2 weeks, the 1 month, and the 2 months before admission were an average exposure on days 14, 15, and 16; 29, 30, and 31; and 59, 60, and 61, respectively. The months of admissions were divided into 4 seasons: winter (December–February), spring (March–May), summer (June–August), and fall (September–November).
Environmental Data
Because it was impossible to identify individual exposures, exposure to citywide pollutant levels was obtained as a surrogate. All of the patients reported a San Francisco zip code at the time of hospitalization. Data from the US Census showed that 95% of San Francisco residents live and work in the same locale/zip code; thus, exposure was likely not due to site of residence or work outside of San Francisco. Pollutant data from all of the Environmental Protection Agency monitoring stations in San Francisco were considered in calculating the mean exposure levels of each pollutant. All stations were within 3 miles of SFGH. However, only 1 station had complete daily data from 1997 to 2008, and therefore that station was used to assign patients their exposure levels. Climatic data were obtained from the Weather Warehouse [33], an open-source collection of historical weather information. The same method as above was used to estimate patients' exposure to citywide climatic factors. But here we considered stations within 18 miles of SFGH. The rationale for using weather centers within 18 miles of SFGH was based on the availability of complete climate data from these centers, the likelihood of patients with PcP admitted to SFGH living and working within this radius, and also the lack of a closer weather center.
Statistical Analysis
We first characterized HIV-positive patients who were hospitalized with a diagnosis of PcP for the first time using medians (with interquartile range [IQR]) or counts (with percentages of total) to describe continuous and discrete characteristics, respectively. All environmental factors, except temperature and ozone, varied greatly across dates of measurements, so they were log-transformed.
Conditional logistic regression was used to evaluate the effect of each environmental factor on PcP hospitalization. For each factor, the odds of PcP hospitalization with respect to an increase in the average level of the factor around the date of hospitalization was obtained (case) and compared to change in the factor when PcP hospitalization did not occur (control). Odds ratios were estimated for a 2.8-unit increase in temperature and ozone, and a 1-unit increase in log–transformed values of the other environmental factors. The linearity of the logistic regression model for measuring the association between PcP hospitalization and each environmental factor was tested by assuming a generalized additive model, with a “smoother” to fit a restricted cubic spline function. Forward stepwise regression was used to combine environmental factors that were significantly related to PcP hospitalization (P < .15) in a multivariate regression model. SAS for Windows, version 9.2 (SAS Institute, Cary, North Carolina) was used to carry out all statistical analyses, and a 5% significance level was assumed, unless stated otherwise.
Ethics Statement
The study was approved by the institutional review boards of the University of California San Francisco and the University of Cincinnati, and all patients provided written informed consent for participation in the study.
RESULTS
PcP Patient Characteristics
From 1 January 1997 to 31 December 2008, 457 consecutive HIV-positive patients were admitted to SFGH with PcP. At admission, their median age was 40.1 years. The majority were men (89%) and white (48%; Table 1). PcP patients had advanced HIV disease with a median CD4 cell count of 31 cells/µL (IQR, 14–64 cells/µL) and a median HIV load of 1.8 × 105 copies/mL (IQR, 7.3 × 104–3.4 × 105 copies/mL). Only 61 patients (13%) had received PcP prophylaxis within the 3 months before admission.
Table 1.
Characteristics of 457 HIV-Positive Patients With First Episode of Pneumocystis Pneumonia Presenting to San Francisco General Hospital
| Characteristic | No.a | Median (IQR) or % |
|---|---|---|
| Age, years | 457 | 40.1 (35.0–45.4) |
| Male sex | 408 | 89% |
| Race | ||
| Black | 125 | 27% |
| White | 217 | 48% |
| Other | 115 | 25% |
| CD4 cell count, cells/µL | 449 | 31 (14–64) |
| HIV RNA, copies/mL | 430 | 1.8 × 105 (7.3 × 104–3.4 × 105 |
| Receipt of PcP prophylaxis | 61 | 13% |
Abbreviations: HIV, human immunodeficiency virus; IQR, interquartile range; PcP, Pneumocystis pneumonia.
a Numbers vary slightly due to missing data.
Effects of Season on PcP Admissions
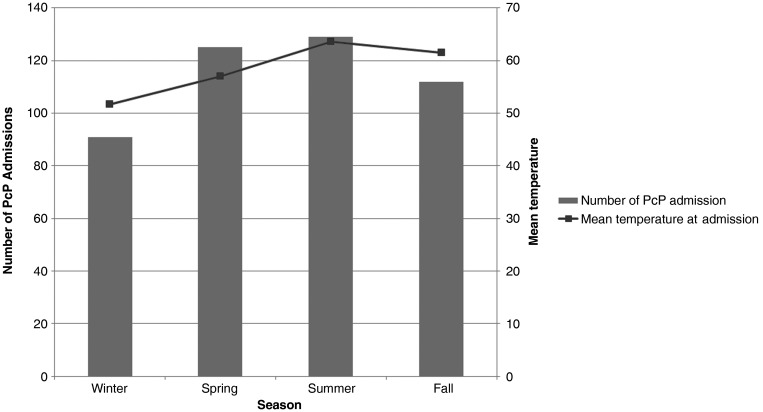
A significant difference in the number of PcP hospital admissions was found across seasons (P < .05). Most admissions occurred in the summer (n = 129), followed by the spring (n = 125). Winter was the season with the fewest number of admissions (n = 91). The peak of PcP admissions in the summer coincided with the peak in mean temperature (Figure 1).
Figure 1.
Total number of Pneumocystis pneumonia admissions by season and mean temperature. Abbreviation: PcP, Pneumocystis pneumonia.
Effects of Environmental Factors on PcP Presentation
Moderate positive correlations were found between SO2 and NO2 and between SO2 and CO (r = 0.66 and r = 0.59, respectively). Ozone was negatively correlated with NO2 and CO (r = −0.63 and r = −0.59, respectively), but CO was positively correlated with NO2 (r = 0.88). Otherwise, the correlations between pairs of the other air pollutants were low.
In single pollutant models using 2 weeks before admission as the control time, temperature and SO2 were significantly associated with PcP hospital admissions (P < .01 and P = .01, respectively). A 5°F increase of temperature was associated with a significant increase in odds of PcP hospitalization (odds ratio [OR], 1.41 [95% confidence interval {CI}, 1.14–1.75]). A 1-unit increase of (log-transformed) SO2 parts per billion (ppb) was associated with a significant increase in PcP hospital admissions (OR, 1.80 [95% CI, 1.15–2.83]). The effects of other variables, including NO2, ozone, PM10, CO, and humidity were not statistically significant (Table 2). Similar results were found when 1 month before admission was used as the control time (Table 3). However, when 2 months before admission were used as the control, only temperature was significantly associated with PcP hospital admissions (Table 4).
Table 2.
Unadjusted and Adjusted Odds Ratios Showing the Association Between Each Environmental Factor and Pneumocystis Pneumonia Hospital Admissions Using 2 Weeks Before Admissions as a Control Time
| Environmental Exposure (Units) | Unadjusted OR (95% CI) | P Value | Adjusted OR (95% CI) | P Value |
|---|---|---|---|---|
| Temperature (°C) | 1.41 (1.14–1.75) | <.01 | 1.47 (1.15–1.88) | <.01 |
| SO2 (ppb) | 1.80 (1.15–2.83) | .01 | 3.04 (1.61–5.73) | <.01 |
| SO2 × CO | … | … | 0.41 (.18–.92) | .03 |
| SO2 (ppb)a | … | … | 2.45 (1.10–5.45) | <.01 |
| SO2 (ppb)b | … | … | 1.34 (1.07–2.60) | <.01 |
| Humidity (%) | 0.61 (.20–1.92) | .40 | 1.06 (.31–3.60) | .92 |
| NO2 (ppb) | 0.91 (.59–1.41) | .68 | 0.49 (.22–1.09) | .08 |
| PM10 (µg/m3) | 0.92 (.70–1.22) | .58 | 0.89 (.65–1.22) | .44 |
| Ozone <20 (ppb) | 1.10 (.73–1.64) | .66 | 1.11 (.90–1.36) | .34 |
| Ozone >20 (ppb) | 0.86 (.44–1.69) | .66 | 0.96 (.68–1.35) | .80 |
| CO (ppb) | 0.77 (.49–1.23) | .27 | 0.46 (.20–1.08) | .07 |
P value tests the significance of the effect of each independent variable on Pneumocystis pneumonia hospital admissions.
Abbreviations: CI, confidence interval; CO, carbon monoxide, NO2, nitrogen dioxide; OR, odds ratio; PM10, particulate matter up to 10 μm in size; ppb, parts per billion; SO2, sulfur dioxide.
a Effect of SO2 at the lower quartile of CO (5.89 ppb).
b Effect of SO2 at the upper quartile of CO (6.59 ppb).
Table 3.
Unadjusted and Adjusted Odds Ratios Measuring the Association Between Each Environmental Factor and Pneumocystis Pneumonia Hospital Admissions Using 1 Month Before Admissions as a Control Time
| Environmental Exposure (Units) | Unadjusted OR (95% CI) | P Value | Adjusted OR (95% CI) | P Value |
|---|---|---|---|---|
| Temperature (°C) | 1.20 (1.01–1.43) | .04 | 1.23 (1.02–1.48) | .03 |
| SO2 (ppb) | 1.62 (1.08–2.44) | .02 | 2.48 (1.25–4.95) | <.01 |
| Humidity (%) | 0.72 (.25–2.07) | .55 | 0.62 ( .16–2.34) | .48 |
| NO2 (ppb) | 0.83 (.55–1.25) | .38 | 0.85 (.39–1.84) | .68 |
| PM10 (µg/m3) | 1.08 (.83–1.40) | .56 | 0.95 (.70–1.30) | .76 |
| Ozone <20 (ppb) | 1.10 (.79–1.53) | .58 | 1.10 (.89–1.36) | .33 |
| Ozone >20 (ppb) | 0.80 (.47–1.37) | .42 | 0.95 (.68–1.34) | .79 |
| CO (ppb) | 0.64 (.41–1.01) | .06 | 0.57 (.27–1.21) | .14 |
P value tests the significance of the effect of each independent variable on Pneumocystis pneumonia hospital admissions.
Abbreviations: CI, confidence interval; CO, carbon monoxide, NO2, nitrogen dioxide; OR, odds ratio; PM10, particulate matter up to 10 μm in size; ppb, parts per billion; SO2, sulfur dioxide.
Table 4.
Unadjusted and Adjusted Odds Ratios Measuring the Association Between Each Environmental Factor and Pneumocystis Pneumonia Hospital Admissions Using 2 Months Before Admissions as a Control Time
| Environmental Exposures (Units) | Unadjusted OR (95% CI) | P Value | Adjusted OR (95% CI) | P Value |
|---|---|---|---|---|
| Temperature (°C) | 1.33 (1.15–1.53) | <.01 | 1.77 (1.33–2.36) | <.01 |
| SO2 (ppb) | 1.32 (.93–1.88) | .12 | 1.33 (.91–1.96) | .14 |
| Humidity (%) | 0.39 (.14–1.08) | .07 | 0.69 (.22–2.13) | .52 |
| NO2 (ppb) | 0.90 (.65–1.25) | .54 | 1.00 (.52–1.94) | .99 |
| PM10 (µg/m3) | 0.92 (.70–1.20) | .52 | 0.84 (.62–1.13) | .25 |
| Ozone <20 (ppb) | 1.02 (.98–1.05) | .34 | 1.18 (.96–1.46) | .12 |
| Ozone >20 (ppb) | 0.98 (.93–1.03) | .40 | 0.87 (.64–1.20) | .40 |
| CO (ppb) | 0.80 (.56–1.16) | .24 | 0.49 (.23–1.04) | .06 |
P value tests the significance of the effect of each independent variable on Pneumocystis pneumonia hospital admissions.
Abbreviations: CI, confidence interval; CO, carbon monoxide, NO2, nitrogen dioxide; OR, odds ratio; PM10, particulate matter up to 10 μm in size; ppb, parts per billion; SO2, sulfur dioxide.
In a multivariate analysis using 2 weeks before admission as the control time, the independent effects of temperature and SO2 were significantly associated with PcP hospital admissions. However, the effect of increasing SO2 on PcP hospital admissions appeared to be attenuated by increasing CO. The interaction between SO2 and CO was statistically significant (P = .03). The magnitude of the effect of SO2 on PcP admissions varied at different levels of CO. At the lower quartile of CO (5.89 ppb), the effect of SO2 was higher (OR, 2.45 [95% CI, 1.10–5.45]), but at the upper quartile of CO (6.59 ppb), the effect of SO2 was lower (OR, 1.34 [95% CI, 1.07–2.60]; Table 2). The interaction between temperature and CO was not significant. When 1 month before admission was used as the control time, the effects of temperature and SO2 remained significant, but the effect of the interaction between SO2 and CO was no longer significant (Table 3). Only temperature appeared to have a significant association with likelihood of PcP hospital admission when 2 months before admission was used as the control time (Table 4).
DISCUSSION
The present study suggests that season, temperature, and SO2 were significant risk factors for admission of HIV-positive patients with PcP to SFGH. Additionally, CO appears to have modified the effects of SO2 on these admissions. Thus, the identification of both climatological and air pollution constituents associated with the development of PcP is a novel and important observation.
The seasonality of hospitalizations for PcP and the association with absolute temperature found in this study are consistent with some reports [11, 13, 14] but not others [12, 16]. Differences in patient populations, geography, Pneumocystis genotypes or virulence factors, and study design or data analysis may have contributed to these inconsistent results [11–14, 16]. For example, only one of these reports used regression techniques for data analysis [13]. It is also possible that winter temperatures in one area can be similar to summer temperatures in another area. For example, in one study in London the “peak” summer temperature was 13°C [14], but in Seville, Spain, the “peak” winter temperature was between 12°C and 14°C [16]. The mean summer temperature for San Francisco in the present study was 17.6°C.
The study design used in this report is distinctive because of the novel use of CCD to analyze the effects of climate on PcP hospitalizations. Cross-sectional designs have previously been used to relate the incidence of PcP to climatic factors. CCD used in this study provided several advantages. First, controls were not sampled, thus reducing time and expense and eliminating sources of bias. Second, clinical and demographic characteristics were controlled for by making within-subject comparisons. Third, this method allowed us to control for geographic variations of PcP incidence previously shown in San Francisco [6]. In cross-sectional analysis, care must be taken to control for all confounding and effect modifying factors; yet, if some of the subjects have missing data related to factors that need to be controlled for, the estimate of the effects may become biased. Other differences between this study and other reports involve the use of daily averaged temperature readings (vs monthly averaged) and the use of a citywide average by averaging climatic factors across stations with complete data.
In this study, SO2 was shown to be an independent risk factor for PcP hospitalizations. Some reports have shown that an increase in SO2 level is associated with an increase in hospital admission rates for respiratory diseases such as COPD, asthma, influenza, and community-acquired pneumonia [25], whereas other reports have not [23, 24]. Although it is difficult to explain how SO2 might increase PcP hospital admissions, it is known that exposure to SO2 in humans causes nose and throat irritation, bronchoconstriction, and dyspnea [22]. Exposure to SO2 animal models leads to lung remodeling, mucus hypersecretion, inflammation, airway hyperresponsiveness, and COPD [34, 35]. Exposure to SO2 also impairs alveolar macrophages with a possible reduction in alveolar macrophage chemotactic activity, and causes different types of respiratory tract inflammation [36, 37]. These observations lend support to the hypothesis that SO2 exposure in the patients described in the present study contributed to an impairment of pulmonary defense mechanisms, and aggravated preexisting and evolving PcP symptoms, resulting in these patients seeking medical care.
It is also possible that other factors (besides immunodeficiency) related to HIV-positive patients themselves could have contributed to increased susceptibility to the effects of temperature and SO2 leading to presentation with PcP. HIV-positive patients have an increased frequency of COPD, bacterial pneumonia, pulmonary hypertension, pulmonary fibrosis, and P. jirovecii colonization of the respiratory tract [3–4, 38]. Smoking is a risk factor for Pneumocystis colonization [38]. These features suggest that the environmental factors would have a greater chance to exert their influence on the development of PcP.
An antagonistic relationship between SO2 and CO was found in this study, with CO appearing to attenuate the effects of SO2 when 2 weeks before admission was used as the control. Previous epidemiologic studies have shown that an increase in CO levels was associated with an increase in respiratory disease hospital admissions. Depending on the disease, studies have reported a >4-fold increase in the risk of admissions associated with CO levels [19–21]. In the present study, CO was found to be protective and to reduce the effects of SO2. Unfortunately, it is difficult to find a good explanation for the association. These 2 pollutants have different sources. SO2 is formed after combustion of fuel containing sulfur, and most SO2 in the air in the United States comes from power plants. However, CO is a traffic-related pollutant, and its presence in the air is mostly from motor vehicles [35]. Nevertheless, the differences in sources are not enough to explain the association reported in this study. Although the effects of temperature on air pollution remain a matter of controversy, it has been shown that more sulfates are generated at high temperature, leading to an increased mortality due to respiratory diseases [39]. It is possible that the increase in temperature was associated with persons spending more time outdoors, and hence a greater likelihood of exposure to SO2. We hypothesize that CO prevents or reduces the temperature-induced generation of sulfates resulting in the observed attenuation of SO2 effect, but this requires confirmation.
The nonsignificant effects of the other air pollution parameters such as ozone, CO, NO2, and PM10 on PcP hospital admissions were surprising. Ozone is known to cause changes in lung function that could lead to disease complications and to increased hospital admissions [40]. In fact, studies have shown that ozone and PM10 levels were both associated with hospital admissions for other respiratory diseases [21, 41, 42]. In addition, NO2 has been linked to hospital admissions for respiratory diseases [21, 24, 43]. One possible reason for the lack of association in our study is that San Francisco is among the least polluted cities in the United States and perhaps the levels of these pollutants were below the thresholds that could cause respiratory complications.
This study has several limitations. First, weather stations and air pollution monitoring stations were used to determine individual exposure levels. This method may lead to exposure underestimation because monitoring stations may not provide detailed information about the microclimate in which each patient was living. Second, despite assigning the citywide exposure level to each subject, some of the monitoring stations did not have data for the entire study period of our study. Those stations were excluded from the calculation of the average exposure.
Third, because this is the first report that used CCD and that found effects of specific air pollutants on PcP hospitalization, it is possible that alternative explanations to our results may arise in the future.
Finally, this study was done in a single city with subtle differences in seasons, so the results may not be generalizable to cities with wider seasonal differences.
In conclusion, this study shows that among climatic and ambient air pollutant constituents, temperature and SO2, present at high levels immediately (0–3 days) prior to admission appeared to be associated with the likelihood of being admitted with PcP among HIV-positive patients living in San Francisco. Additional multicenter studies are needed to identify if these factors are also predictors of HIV-associated PcP admissions in other geographic locations and in other immunocompromised groups. Animal studies are also needed to better understand the biological mechanisms behind the impact of climatic and air pollution on PcP occurrence.
Notes
Acknowledgments. We thank Ralph Buncher and Ranjan Deka (who received no remuneration) from the University of Cincinnati for their assistance.
Financial support. This work was supported by the National Institutes of Health (K24 HL087713 and RO1 HL090335) and the Department of Veterans Affairs.
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Buchacz K, Baker RK, Palella FJ, Jr, et al. AIDS-defining opportunistic illnesses in US patients, 1994–2007: a cohort study. AIDS. 2010;24:1549–59. doi: 10.1097/QAD.0b013e32833a3967. [DOI] [PubMed] [Google Scholar]
- 2.Morris A, Lundgren JD, Masur H, et al. Current epidemiology of Pneumocystis pneumonia. Emerg Infect Dis. 2004;10:1713–20. doi: 10.3201/eid1010.030985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morris A, Alexander T, Radhi S, et al. Airway obstruction is increased in Pneumocystis-colonized human immunodeficiency virus-infected outpatients. J Clin Microbiol. 2009;47:3773–6. doi: 10.1128/JCM.01712-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morris AM, Huang L, Bacchetti P, et al. Permanent declines in pulmonary function following pneumonia in human immunodeficiency virus-infected persons. The Pulmonary Complications of HIV Infection Study Group. Am J Respir Crit Care Med. 2000;162:612–6. doi: 10.1164/ajrccm.162.2.9912058. [DOI] [PubMed] [Google Scholar]
- 5.Sassi M, Ripamonti C, Mueller NJ, et al. Outbreaks of Pneumocystis pneumonia in 2 renal transplant centers linked to a single strain of pneumocystis: implications for transmission and virulence. Clin Infect Dis. 2012;54:1437–44. doi: 10.1093/cid/cis217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morris AM, Swanson M, Ha H, Huang L. Geographic distribution of human immunodeficiency virus-associated Pneumocystis carinii pneumonia in San Francisco. Am J Respir Crit Care Med. 2000;162:1622–6. doi: 10.1164/ajrccm.162.5.2002065. [DOI] [PubMed] [Google Scholar]
- 7.Dohn MN, White ML, Vigdorth EM, et al. Geographic clustering of Pneumocystis carinii pneumonia in patients with HIV infection. Am J Respir Crit Care Med. 2000;162:1617–21. doi: 10.1164/ajrccm.162.5.9707101. [DOI] [PubMed] [Google Scholar]
- 8.Navin TR, Rimland D, Lennox JL, et al. Risk factors for community-acquired pneumonia among persons infected with human immunodeficiency virus. J Infect Dis. 2000;18:158–64. doi: 10.1086/315196. [DOI] [PubMed] [Google Scholar]
- 9.Icenhour CR, Arnold J, Medvedovic M, Cushion MT. Competitive coexistence of two Pneumocystis species. Infect Genet Evol. 2006;6:177–86. doi: 10.1016/j.meegid.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 10.Miller RF, Evans HE, Copas AJ, Cassell JA. Climate and genotypes of Pneumocystis jirovecii. Clin Microbiol Infect. 2007;13:445–8. doi: 10.1111/j.1469-0691.2006.01641.x. [DOI] [PubMed] [Google Scholar]
- 11.Hoover DR, Graham NM, Bacellar H, et al. Epidemiologic patterns of upper respiratory illness and Pneumocystis carinii pneumonia in homosexual men. Am Rev Respir Dis. 1991;144:756–9. doi: 10.1164/ajrccm/144.4.756. [DOI] [PubMed] [Google Scholar]
- 12.Lubis N, Baylis D, Short A, et al. Prospective cohort study showing changes in the monthly incidence of Pneumocystis carinii pneumonia. Postgrad Med J. 2003;79:164–6. doi: 10.1136/pmj.79.929.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sing A, Schmoldt S, Laubender RP, Heesemann J, Sing D, Wildner M. Seasonal variation of Pneumocystis jirovecii infection: analysis of underlying climatic factors. Clin Microbiol Infect. 2009;15:957–60. doi: 10.1111/j.1469-0691.2009.02804.x. [DOI] [PubMed] [Google Scholar]
- 14.Miller RF, Grant AD, Foley NM. Seasonal variation in presentation of Pneumocystis carinii pneumonia. Lancet. 1992;339:747–8. doi: 10.1016/0140-6736(92)90650-r. [DOI] [PubMed] [Google Scholar]
- 15.Walzer PD, Perl DP, Krogstad DJ, Rawson PG, Schultz MG. Pneumocystis carinii pneumonia in the United States: epidemiologic, diagnostic, and clinical features. Ann Intern Med. 1974;80:83–93. doi: 10.7326/0003-4819-80-1-83. [DOI] [PubMed] [Google Scholar]
- 16.Varela JM, Regordan C, Medrano FJ, et al. Climatic factors and Pneumocystis jiroveci infection in southern Spain. Clin Microbiol Infect. 2004;10:770–2. doi: 10.1111/j.1469-0691.2004.00937.x. [DOI] [PubMed] [Google Scholar]
- 17.Palepu A, Sun H, Kuyper L, Schechter MT, O'Shaughnessy MV, Anis AH. Predictors of early hospital readmission in HIV-infected patients with pneumonia. J Gen Intern Med. 2003;18:242–7. doi: 10.1046/j.1525-1497.2003.20720.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Walzer PD, Evans HE, Copas AJ, Edwards SG, Grant AD, Miller RF. Early predictors of mortality from Pneumocystis jirovecii pneumonia in HIV-infected patients: 1985–2006. Clin Infect Dis. 2008;46:625–33. doi: 10.1086/526778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fusco D, Forastiere F, Michelozzi P, et al. Air pollution and hospital admissions for respiratory conditions in Rome, Italy. Eur Respir J. 2001;17:1143–50. doi: 10.1183/09031936.01.00005501. [DOI] [PubMed] [Google Scholar]
- 20.Sunyer J, Anto JM, Murillo C, Saez M. Effects of urban air pollution on emergency room admissions for chronic obstructive pulmonary disease. Am J Epidemiol. 1991;134:277–86. doi: 10.1093/oxfordjournals.aje.a116081. discussion 87–9. [DOI] [PubMed] [Google Scholar]
- 21.Linn WS, Szlachcic Y, Gong H, Jr, Kinney PL, Berhane KT. Air pollution and daily hospital admissions in metropolitan Los Angeles. Environ Health Perspect. 2000;108:427–34. doi: 10.1289/ehp.00108427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kampa M, Castanas E. Human health effects of air pollution. Environ Pollut. 2008;151:362–7. doi: 10.1016/j.envpol.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 23.Neupane B, Jerrett M, Burnett RT, Marrie T, Arain A, Loeb M. Long-term exposure to ambient air pollution and risk of hospitalization with community-acquired pneumonia in older adults. Am J Respir Crit Care Med. 2010;181:47–53. doi: 10.1164/rccm.200901-0160OC. [DOI] [PubMed] [Google Scholar]
- 24.Walters S, Phupinyokul M, Ayres J. Hospital admission rates for asthma and respiratory disease in the West Midlands: their relationship to air pollution levels. Thorax. 1995;50:948–54. doi: 10.1136/thx.50.9.948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martins LC, Latorre Mdo R, Cardoso MR, Goncalves FL, Saldiva PH, Braga AL. [Air pollution and emergency room visits due to pneumonia and influenza in Sao Paulo, Brazil] Rev Saude Publica. 2002;36:88–94. doi: 10.1590/s0034-89102002000100014. [DOI] [PubMed] [Google Scholar]
- 26.Zanobetti A, Schwartz J. Air pollution and emergency admissions in Boston, MA. J Epidemiol Community Health. 2006;60:890–5. doi: 10.1136/jech.2005.039834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carracedo-Martinez E, Taracido M, Tobias A, Saez M, Figueiras A. Case-crossover analysis of air pollution health effects: a systematic review of methodology and application. Environ Health Perspect. 2010;118:1173–82. doi: 10.1289/ehp.0901485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Medina-Ramon M, Zanobetti A, Schwartz J. The effect of ozone and PM10 on hospital admissions for pneumonia and chronic obstructive pulmonary disease: a national multicity study. Am J Epidemiol. 2006;163:579–88. doi: 10.1093/aje/kwj078. [DOI] [PubMed] [Google Scholar]
- 29.Maclure M. The case-crossover design: a method for studying transient effects on the risk of acute events. Am J Epidemiol. 1991;133:144–53. doi: 10.1093/oxfordjournals.aje.a115853. [DOI] [PubMed] [Google Scholar]
- 30.Huang L, Stansell JD. AIDS and the lung. Med Clin North Am. 1996;80:775–801. doi: 10.1016/s0025-7125(05)70467-3. [DOI] [PubMed] [Google Scholar]
- 31.Fei MW, Sant CA, Kim EJ, et al. Severity and outcomes of Pneumocystis pneumonia in patients newly diagnosed with HIV infection: an observational cohort study. Scand J Infect Dis. 2009;41:672–8. doi: 10.1080/00365540903051633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kovacs JA, Hiemenz JW, Macher AM, et al. Pneumocystis carinii pneumonia: a comparison between patients with the acquired immunodeficiency syndrome and patients with other immunodeficiencies. Ann Intern Med. 1984;100:663–71. doi: 10.7326/0003-4819-100-5-663. [DOI] [PubMed] [Google Scholar]
- 33.Weather Warehouse. http://weather-warehouse.com/index.html . 2009. [Google Scholar]
- 34.Wagner U, Staats P, Fehmann HC, Fischer A, Welte T, Groneberg DA. Analysis of airway secretions in a model of sulfur dioxide induced chronic obstructive pulmonary disease (COPD) J Occup Med Toxicol. 2006;1:12. doi: 10.1186/1745-6673-1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen TM, Gokhale J, Shofer S, Kuschner WG. Outdoor air pollution: nitrogen dioxide, sulfur dioxide, and carbon monoxide health effects. Am J Med Sci. 2007;333:249–56. doi: 10.1097/MAJ.0b013e31803b900f. [DOI] [PubMed] [Google Scholar]
- 36.Knorst MM, Kienast K, Gross S, Fries B, Muller-Quernheim J, Ferlinz R. Chemotactic response of human alveolar macrophages and blood monocytes elicited by exposure to sulfur dioxide. Res Exp Med (Berl) 1996;196:127–35. doi: 10.1007/BF02576834. [DOI] [PubMed] [Google Scholar]
- 37.Knorst MM, Kienast K, Muller-Quernheim J, Ferlinz R. Effect of sulfur dioxide on cytokine production of human alveolar macrophages in vitro. Arch Environ Health. 1996;51:150–6. doi: 10.1080/00039896.1996.9936009. [DOI] [PubMed] [Google Scholar]
- 38.Morris A, Norris KA. Colonization by Pneumocystis jirovecii and its role in disease. Clin Microbiol Rev. 2012;25:297–317. doi: 10.1128/CMR.00013-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Park AK, Hong YC, Kim H. Effect of changes in season and temperature on mortality associated with air pollution in Seoul, Korea. J Epidemiol Community Health. 2011;65:368–75. doi: 10.1136/jech.2009.089896. [DOI] [PubMed] [Google Scholar]
- 40.Spektor DM, Lippmann M, Thurston GD, et al. Effects of ambient ozone on respiratory function in healthy adults exercising outdoors. Am Rev Respir Dis. 1988;138:821–8. doi: 10.1164/ajrccm/138.4.821. [DOI] [PubMed] [Google Scholar]
- 41.Burnett RT, Brook JR, Yung WT, Dales RE, Krewski D. Association between ozone and hospitalization for respiratory diseases in 16 Canadian cities. Environ Res. 1997;72:24–31. doi: 10.1006/enrs.1996.3685. [DOI] [PubMed] [Google Scholar]
- 42.Wordley J, Walters S, Ayres JG. Short term variations in hospital admissions and mortality and particulate air pollution. Occup Environ Med. 1997;54:108–16. doi: 10.1136/oem.54.2.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Anderson HR, Spix C, Medina S, et al. Air pollution and daily admissions for chronic obstructive pulmonary disease in 6 European cities: results from the APHEA project. Eur Respir J. 1997;10:1064–71. doi: 10.1183/09031936.97.10051064. [DOI] [PubMed] [Google Scholar]