Abstract
Transmission of highly pathogenic avian influenza (HPAI) between birds and humans is an ongoing threat that holds potential for the emergence of a pandemic influenza strain. A major barrier to an effective vaccine against avian influenza has been the generally poor immunopotency of many of the HPAI strains coupled with the manufacturing constraints employing conventional methodologies. Fusion of flagellin, a toll-like receptor-5 ligand, to vaccine antigens has been shown to enhance the immune response to the fused antigen in preclinical studies. Here, we have evaluated the immunogenicity and efficacy of a panel of flagellin-based hemagglutinin (HA) globular head fusion vaccines in inbred mice. The HA globular head of these vaccines is derived from the A/Vietnam/1203/04 (VN04; H5N1) HA molecule. We find that replacement of domain D3 of flagellin with the VN04 HA globular head creates a highly effective vaccine that elicits protective HAI titers which protect mice against disease and death in a lethal challenge model.
Keywords: Avian flu vaccine, HA globular head, Prokaryotic production
1. Introduction
Influenza A viruses are responsible for recurrent seasonal epidemics as well as periodic global pandemics associated with acute respiratory disease and death [1]. The epidemiological success of these viruses is due in large part to a segmented genome which allows them to evolve by exchange, or reassortment, of gene segments between different influenza A strains. When reassortment involves an exchange of one of the major surface proteins, it is known as antigenic shift and the virus that emerges usually presents the human immune system with such a novel antigenic experience that it results in high morbidity and/or mortality [2]. The most clinically profound antigenic shifts involve the key protective antigen, hemagglutinin (HA). Thus, novel influenza A subtypes arising from antigenic shift, particularly those involving HA, can lead to severe pandemics due to the absence of pre-existing protective immunity in individuals and the population as a whole [1,3].
Highly virulent influenza A variants often emerge in birds or swine and then cross species barriers to infect humans [3–9]. Currently, the highly pathogenic avian influenza (HPAI) viruses circulating in Asia are regarded as having the potential for crossing the species barrier and causing a pandemic [10–12]. In 1997, the first human case involving HPAI of the H5N1 subtype was reported in Southeast Asia. Subsequently, over 360 human cases of HPAI of the H5 subtype, with a case-fatality rate over 60%, have been reported [13–15]. Although poultry-to-human transmission remains the pre-dominant means of transmission, public health risk may increase significantly if the human-to-human barrier is overcome by a reassortant virus.
Vaccines are central to pandemic preparedness. However, two barriers to the development and production of vaccines that address global pandemic influenza needs have emerged. First, the immunological potency of the HA associated with pandemic strains is significantly less than that observed for the seasonal human influenza strains [16,17]. Second, existing influenza manufacturing processes that use either eggs or eukaryotic cell culture are inefficient and require large centralized, committed manufacturing facilities that are not amenable to the rapid production of stockpiles sufficient for national or global requirements [18–20]. We have developed an approach that addresses these barriers by linking the Toll-like receptor (TLR) 5 agonist, flagellin, to a self-sufficient protective subunit of HA.
Toll-like receptors (TLRs) are expressed on various cell types, including professional antigen presenting cells, where they act as primary sensors of microbial infection and then activate signaling cascades that lead to the induction of immune responses [21,22]. It is well established that physical linkage of TLR ligands and vaccine antigens enhances the immunopotency of the linked antigen [23]. In previous studies, we have demonstrated that physical linkage of vaccine antigens to flagellin generates a significantly more potent vaccine than simple mixing of antigen and flagellin [24–26].
More recently, we have used this approach to generate novel influenza vaccines that fuse the globular head of HA with flagellin [27]. The globular head domain spans the majority of the neutralizing epitopes in HA and, in the context of the flagellin fusion protein, stably refolds to faithfully form these conformationally sensitive epitopes. These fusion proteins are immunologically potent vaccines that can be efficiently manufactured at scales to meet global needs using standard E. coli fermentation systems. We have now extended this approach to the development and evaluation of several prototypic pandemic vaccines based on the A/Vietnam/1203/2004 H5 strain (VN04). We find that replacement of domain D3 of flagellin with the globular head domain of the Vietnam HA leads to the generation of a highly effective vaccine in the mouse lethal challenge model.
2. Materials and methods
2.1. Tissue and egg culture
The Madin-Darby canine kidney (MDCK) and African green monkey kidney (Vero) cell lines (American Type Culture Collection, Manassas, VA) were maintained in minimal essential medium (MEM) supplemented with 10% fetal bovine serum and antibiotics. SPAFAS Specific Pathogen Free premium eggs were supplied by Charles River Laboratories (Wilmington, MA).
2.2. Viruses
Animal infections and viral assays were performed with influenza A/Vietnam/1203/04 (Influenza Laboratory, U.S. Centers for Disease Control and Prevention, Atlanta, GA) using virus stock obtained by cultivation for 20–36 h at 37 °C in embryonated chicken eggs (Charles River Laboratories, Wilmington, MA). Aliquots of harvested virus were stored at −80 °C until use. Viral stock and inoculum dose was determined by TCID50 (tissue culture infectious dose) assay. All work with this virus isolate was approved by institutional and federal agencies (CDC/USDA) and was performed in the Robert E. Shope Laboratory at BSL-4 at the University of Texas Medical Branch (Galveston, TX).
2.3. TCID50 assay
Serial 10-fold dilutions of the virus stock or of a 10% tissue homogenate was prepared in MEM without serum. MDCK cells were grown to confluence in 96-well tissue culture plates, washed twice with 100 μl of DPBS, followed by inoculation of 100 μl of each virus dilution of virus into four replicate wells, or, as negative control, DPBS. Plates were incubated for 90 min at 37 °C, 5% CO2, after which an additional 100 μl of MEM was added to each well. Plates were incubated for 4 days at 37 °C, 5% CO2. HA assay [28] was performed by removing 50 μl of supernatant from each well and transferring it to a 96-well plate, followed by addition of 50 μl per well of a 0.5% solution of horse erythrocytes suspended in DPBS with Ca2+ and Mg2+. Erythrocytes were allowed to settle and hemagglutination was documented for each replicate. Virus concentration of stocks for infection was determined as TCID50 per ml. For organ titrations, infectious virus titers were expressed as TCID50 per gram (g) of tissue [28].
2.4. Vaccine design and formulation
2.4.1. Cloning of recombinant HA genes
E. coli
the codon-optimized synthetic genes of the HA globular head domain of influenza A/Vietnam/1203/04 were fused directly to the C-terminus of the full-length sequence of Salmonella typhimuriumfljB (flagellin phase 2), STF2 (DNA2.0 Inc., Menlo Park, CA) or fused to replace either the domain D3 of STF2 (aa191–aa292) or domain D0 of STF2 (aa1–aa46 and aa465–aa506, HA1-2 fused to aa464). In C-terminal fusion, the last amino acid of flagellin, R506, was mutated to A506 to reduce proteolytic breakdown. The resulting constructs were cloned into the pET24a vectors to generate the constructs STF2.HA1-2 (VN), STF2R3.HA1-2 (VN) and STF2R0.HA1-2 (VN). The plasmids were used to transform BLR (DE3) cells (Novagen, San Diego, CA; Cat #69053) to generate working cell banks.
Baculovirus
the synthetic genes encoding HA0 or HA1-1 (VN) were codon-optimized for Baculovirus expression (DNA2.0 Inc., Menlo Park, CA) and cloned into pAcGP67A™ and pAcGP67B™ (BD Biosciences) vectors, respectively. A C-terminal thrombin cleavage site, a foldon sequence and 6xHis tag were fused to the HA0 carboxyl terminus to facilitate trimer formation and purification [29]. The recombinant baculoviruses were generated by co-transfecting Sf9 cells with the recombinant plasmids and BD BaculoGold™ DNA following standard Baculovirus Expression protocol (Invitrogen, Carlsbard, CA).
2.4.2. Expression and purification of HA globular head-flagellin fusion proteins
E. coli expressed proteins
flagellin fusion proteins were manufactured utilizing a fed-batch fermentation process in E. coli. After complete exhaustion of the available glucose during the batch phase, 4 l of enriched synthetic feed media was pumped at a controlled rate over an additional 10.5 h (for a total process time was 30.3 h). Expressions of the target protein were induced with 2.1 mM IPTG (final concentration). Cells were pelleted by centrifugation and cell paste was stored at −20 °C. Cell paste was thawed and diluted to 15% solids in 50 mM Tris 25 mM NaCl (pH 8). The suspension was homogenized three times under 12k PSI. STF2.HA1-2 and STF2R0.HA1-2 were located in both supernatant and pellet. Only supernatant was processed. The majority of STF2R3.HA1-2 was found in the pellet. Only the inclusion body was processed. For the supernatant process, protein fractions containing the fusion protein were precipitated by either 10% polyethylene glycol (PEG) 3350 or by 4 M (NH4)2SO4. The pellets were dissolved in 8 M urea at pH 4 to solubilize the target protein. Soluble proteins were extracted in the supernatant phase by centrifugation. Supernatants were bound to a CEX column (Tosoh SP650 M) in 6 M urea and low salt. The target proteins were eluted by NaCl step elution. The collected proteins were refolded by rapid dilution using 20 mM Tris, 0.5 M urea, 0.1 M trehalose, 2 mM CaCl2, 3 mM cysteine, 0.3 mM cystine, 1 mM EDTA, 0.1% PS-80, pH 8.0 with constant stirring overnight. The refolded proteins were concentrated to 1 l and the buffer exchanged using 50 mM Tris, 0.05% PS80, 0.1 M trehalose (pH 8). Q anion exchange chromatography was performed to remove remaining impurities. High protein containing, Q eluate peak fractions were selected for further processing. Size exclusion chromatography was performed as a final purification step to isolate the purified monomeric form of the target proteins. For the pellet process, the inclusion body was washed with 1% Triton X-100 and solubilized with 8 M urea. The protein was refolded by the rapid dilution using the same condition. Further purification follows the same steps as the supernatant process. Final bulk protein was stored at −70 °C as 1 ml aliquots. Residual endotoxin was assayed by standard Chromogenic Limulus Amebocyte Lysate assay (Lonza, Walkersville, MD) as directed by the manufacturer.
Baculovirus expressed proteins
for the 6xHis tagged proteins, the nickel chelating column was employed. Protein was loaded to a Ni-NTA column equilibrated in 20 mM Tris, pH 8, 0.2 M NaCl and eluted in a gradient of 0–0.5 M imidazole. The target protein was further purified by size exclusion column (10/24 GL, GE) using F147 buffer (10 mM histidine, 10 mM Tris, 150 mM NaCl, 5% trehalose, 0.02% PS80, 0.1 mM EDTA, 0.5% ethanol, pH 7.0). Aliquoted protein solution was stored at −80 °C. The foldon motif and the 6xHis tag of HA0 (VN) were further removed by treating with thrombin (1:20 (w/w)) overnight at room temperature. The digestion mixture was then passed sequentially through Ni column and benzamidine column (GE Healthcare) to remove His-tagged fragments, uncleaved proteins and thrombin. The flow through was concentrated and applied to Superdex 200 column (10/24 GL, GE Healthcare) using F147 buffer. Aliquoted protein solution was stored at −80 °C.
2.5. Characterization of flagellin–HA globular head fusion proteins
2.5.1. Western blot
E. coli expressed, purified STF2.HA1-2 (VN), STF2R0.HA1-2 (VN) and STF2R3.HA1-2 (VN) fusion proteins were resolved via SDS-PAGE and Western blot was performed using rabbit polyclonal antibody specific for flagellin (Covance Research Products, Denver, PA) or sheep hyperimmune serum raised against influenza A/Vietnam/1203/2004 (VN04) virus (provided by the National Institute for Biological Standard and Control (NIBSC, UK)).
2.5.2. TLR5 bioassay
TLR5-specific activity of fusion proteins was evaluated by measuring induction of IL-8 production by HEK 293 cells (ATCC). Cells were cultured in 96-well microtiter plates (Costar) at a seeding density of 3–5 × 104 cells in 100 μl/well in DMEM medium supplemented with 10% FCS and antibiotics. The next day, cells were treated for 5 h with serial dilutions of test proteins starting at 5 μg/ml. At the completion of the assay, supernatants were harvested and IL-8 expression was evaluated by ELISA (Invitrogen, Carlsbad, CA). OD450 was measured on a microplate spectrophotometer (Molecular Devices-MDS, Sunnyvale, CA).
2.6. Immunization protocol
2.6.1. Animals
All animal studies were approved by the Institutional Animal Care and Use Committee at the University of Texas Medical Branch or Princeton University and were carried out according to NIH guidelines. BALB/c mice were purchased from Harlan (Indianapolis, IN). Vaccination and implantation of transponders for telemetric temperature recording was carried out in the animal biosafety level (ABSL)-2 facility, as previously reported [30,31]. H5N1 virus infection was performed in the ABSL-4 facility.
2.6.2. Vaccination
Six-week-old female BALB/c mice (Harlan) were vaccinated sub-cutaneously (s.c.) with two or three doses of vaccine (day −28, −14) or (−42, −28, −14) in 100 μl of vehicle (F147). The animals were bled 7 or 12 days following the last vaccination. Seroconversion was then evaluated via HAI assay (see Section 2.8). For efficacy studies, H5N1 infection was subsequently performed (see Section 2.6.3). Clinical observations of disease development and mortality were monitored daily during the pre-vaccination period, as follows: day −28 to −1 (two vaccine dose trials) or day −42 to −1 (three vaccine dose trials). The weights were recorded periodically, as indicated in figure legends.
2.6.3. Challenge
Prior to virus infection, anesthesia was performed using 5% isofluorane. The mice were then infected intranasally (i.n.) with influenza A/Vietnam/1203/04 at a dose determined in units of TCID50 per animal of H5N1 in 40 μl of PBS (day 0). Back-titration of the inoculum was performed to determine the delivered dose (see Section 2.3). Clinical observations of disease development and mortality were monitored daily during the pre-vaccination (see Section 2.6.2) or post-challenge period (day 0 to day +20–21) and weights were recorded. All animals that developed paralysis and were not able to reach feeders or water bottles were euthanized. Statistical analysis of survival for all groups over the indicated period was performed using logrank test at a significant level of α < 0.05 in GraphPad® Prism (San Diego, CA). For pairwise comparison of the survival of treated and untreated (or mock-treated) groups Fisher’s Exact Test was performed at a significant level of α < 0.05 in GraphPad® Prism. The p-values (logrank and Fisher’s Exact Test) are provided in figure legend. The level of infectious virus in organs was evaluated following preparation of a 10% homogenate (see Section 2.3).
2.6.4. Clinical disease definitions
Standardized data reporting by uniformly trained veterinary technicians was performed daily with data linked to BSDS animal identification (ID) numbers and parallel weight telemetric measurements. Outcomes monitored were death, and the development of encephalitis or paralysis using the following definitions: encephalitis, development of discoordination, ataxia or transient seizures with retention of the ability to drink and feed; paralysis, hind limb (hemiplegic) or quadriplegic paralysis with the inability to reach the feeder or water bottle.
2.7. H5-ELISA
ELISA plates were coated with each of the HA proteins at the indicated concentrations in PBS overnight at 4 °C, blocked with 200–300 μl/well of Assay Diluent Buffer (ADB; BD Pharmingen) for 2–3 h at 23–27 °C. After incubation with the indicated detection antibodies, HRP-labeled goat anti-mouse antibody (Jackson Immunochemical) diluted in ADB was added and the plates were incubated at 23–27 °C for 1–2 h. All washes between reagent addition steps were performed 3 times with 1× PBS/0.05% Tween-20. After adding TMB Ultra substrate (Pierce) and monitoring color development, the reaction was stopped with 1 M H2SO4 and OD450 was measured on a microplate spectrophotometer.
2.8. Hemagglutination inhibition (HAI) test
HI antibody titer against influenza A/Vietnam/1203/2004 (VN04) was measured by a standard method at BSL3 facility (Southern Research Institute) [28], as follows. Antigen was prepared and the total HA units of the stock were determined as described for hemagglutination assay [32]. Sera were treated with receptor destroying enzyme, diluted, and incubated with 4 HA units (HAU) of influenza A/Vietnam/1203/2004 virus in 25 μl for 45 min at room temperature. Horse red blood cells (1%) were added (50 μl/well), mixed briefly, and incubated for 1 h at room temperature. The HAI titers of serum samples were reported as the reciprocal of the highest dilution at which hemagglutination was completely inhibited.
3. Results
3.1. Rationale for vaccine designs
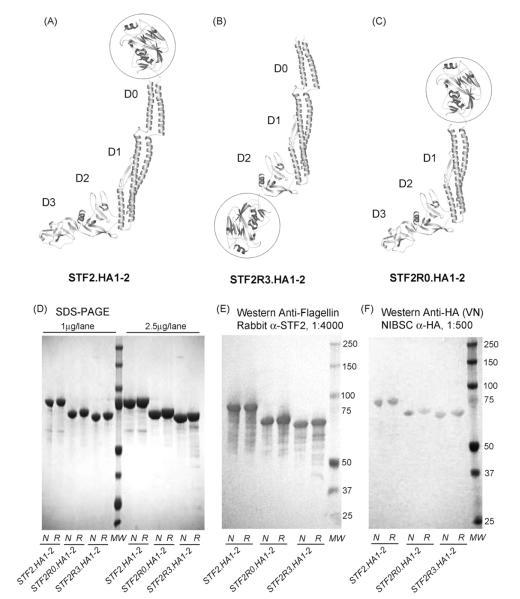
The HA globular head domain contains the cell surface receptor binding site and the majority of the neutralizing antibody epitopes[23,33]. We have recently demonstrated that when the H1 HA globular head is fused to the C-terminus of flagellin, the recombinant influenza vaccines elicited HA specific antibody responses and protected mice from the lethal challenge [27]. Here, we have developed a similar approach to pandemic vaccines by generating a similar subunit vaccine which encompasses the neutralizing epitopes of the A/Vietnam/1203/2004 HA globular head. The domain boundary was placed between residues G62 and E284 to generate the HA subunit designated as HA1-2. The HA1-2 subunit was further fused to the C-terminus of Salmonella typhimurium flagellin type 2 (STF2) to form STF2.HA1-2 (VN) (Fig. 1A, ribbon diagram of C-terminal fusion).
Fig. 1.
VN04 vaccine construct design, protein purification and functional assessment. Schematic representation of (A) STF2.HA1-2 (VN), (B) STF2R3.HA1-2 (VN) and (C) STF2R0.HA1-2 (VN) constructs are shown in ribbon diagram. The globular head of HA is circled and the domains of flagellin are labeled as D0, D1, D2 and D3. Each of the VN04 vaccines was expressed in E. coli, purified, resolved via SDS-PAGE (1 and 2.5 μg per lane, respectively, (D) and transferred to membranes and probed with: (1) rabbit polyclonal antibodies specific for flagellin (E) 1:4000 dilution) or (2) sheep hyperimmune serum raised against purified VN04 antigens from NIBSC (1F, 1:500). All three proteins were homogeneous in gel and further assessed as 99% pure by HPLC reverse phase chromatograpghy. N; non-reducing. Sample was not treated with reducing reagent DTT; R: reducing. Sample was treated with 100 mM DTT and boiled before loading on gel.
In an alternative series of constructs, the globular head of HA was placed in the hypervariable “hinge region” of flagellin by replacing domain D3 (amino acid 191–291, R3 configuration). Instead of tethering at one end to the flagellin as the C-terminal fusion, replacement (R3) tethers both ends of globular head to the flagellin. The potential advantage of the R3 configuration is that it limits the mobility of the HA globular head so that the antigen may be better presented in a more native fashion to the antigen recognition site of antibodies. Furthermore, crystallographic and high resolution electron cryomicroscopic models show that the N- and C-terminal peptides of flagellin come together to form a two-stranded coiled-coil that is referred to as the D0 domain [34]. When forming flagella, the D0 domain is highly structured and constitutes the central tube of the flagella while the adjacent D1 domain lines up to form the outer tube. The coiled-coil structure of the D0 domain is well maintained through the extensive intermolecular interactions among adjacent D0 domains and D0 and D1 domains. The model suggests that, without these inter-molecular restrictions, the D0 domain structure is not stable. Vonderviszt et al. further demonstrated that in solution, the D0 domain of the monomeric flagellin is unstructured, leaving roughly 46 residues of N-terminus and 41 residues at the C-terminus as extended flexible peptide [35]. As a result, in the C-terminal fusion configuration, the flexibility of the peptide preceding the fused HA head may allow intra- or inter-molecular interactions that hinder the optimal antigenic presentation of HA globular head. The extent of these inter- or intra-molecular interactions could differ among HA molecules depending on the surface chemistry of the globular head. By contrast, the R3 configuration avoids this potential hindrance. We designated this construct as STF2R3.HA1-2 VN (Fig. 1B). Finally, in a third design also aimed at reducing the potential for intra-molecular interactions, we replaced the flexible D0 domain with the HA globular head to generate STF2R0.HA1-2 (VN) (Fig. 1C).
3.2. Expression and characterization of the STF2.HA1-2 (VN), STF2R3.HA1-2 (VN) and STF2R0.HA1-2 (VN) vaccines
The STF2.HA1-2 (VN), STF2R3.HA1-2 (VN) and STF2R0.HA1-2 (VN) fusion proteins were expressed using standard E. coli cell culture. All three proteins expressed well following induction. The proteins were denatured and refolded by rapid dilution. Purified proteins were recognized in Western blot both by A/Vietnam/1203/2004 (VN04) sheep hyperimmune sera and the rabbit polyclonal anti-flagellin antibody (Fig. 1D SDS-PAGE, 1E Western-Anti-flagellin and 1F Western-Anti-HA), indicative of fusion integrity.
3.3. Comparative antigenicity of VN vaccine constructs
The relative antigenicity of the VN vaccine constructs was evaluated by ELISA. ELISA plates were coated with increasing concentrations of the different Vietnam protein preparations. Molar equivalents of the different proteins were used. Two proteins, produced using the baculovirus expression system, were included as positive controls. The first of these, HA0, corresponds to the full ecto-domain of the HA protein and the second control, HA1-1, corresponds to the majority of the HA1 peptide. The coated ELISA plates were then probed with convalescent ferret sera obtained from the Centers for Disease Control (CDC). The results are shown in Fig. 2. The strongest reactivity was observed for the two positive control constructs, HA1-1 and HA0. The R3 construct was the next most reactive within the groups. The STF2.HA1-2 (VN) protein reacted relatively poorly with the convalescent sera as did the R0 construct.
Fig. 2.
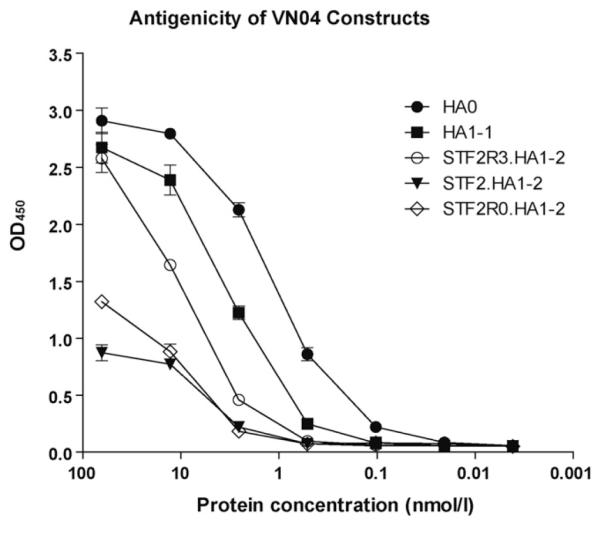
Relative antigenicity of VN04 vaccine constructs. ELISA plates were coated with the indicated molar concentrations of the different proteins. Plates were probed with ferret convalescent serum (1:1000). Mean absorbance with standard deviation for 4 replicate wells are reported.
In a second series of experiments the different Vietnam constructs were probed with a panel of neutralizing monoclonal antibodies specific for the Vietnam HA (Fig. 3, Rockland Immunochemicals, Inc., Gilbertsville, PA). When probed with VN specific monoclonal antibodies, the reactivity of the R3 construct, was comparable to baculovirus produced HA1-1 which reacted similarly to the full-length ecto-domain of HA0 in the direct ELISA (Fig. 2). By contrast, R0 and conventional STF2.HA1-2 reacted poorly with all of monoclonal antibodies that were tested (Fig. 3). These results further suggested that the HA globular head may be better presented in the context of the R3 construct. None of the tested constructs interacted with mAb977, which might be due to the absence of the specific epitope in these globular head subunit constructs.
Fig. 3.
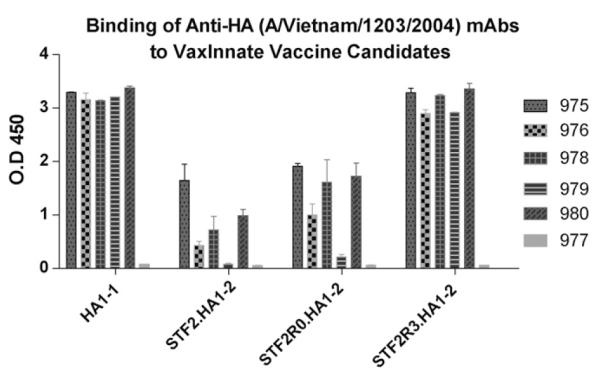
Relative reactivity of monoclonal antibody against various VN04 constructs. ELISA plates were coated with 4 μg/ml of each protein in duplicates overnight, blocked and incubated with a 1:5000 dilution of each of the monoclonal antibodies (Rockland Inc. (500-301-980)) for 2 h at room temperature (one antibody per plate), followed by a 30 min incubation of a 1:10,000 dilution of HRP-goat anti-mouse IgG for 30 min and developed with TMB. Mean absorbance with standard deviation for replicate wells are reported.
3.4. Comparative TLR5 activity of VN vaccine constructs
The ability of flagellin to activate TLR5 was assessed using the in vitro assay described in Section 2. In this assay, the STF2R3.HA1-2 (VN) construct induced strong IL-8 secretion comparable to the STF2 control, which is indicative of potent TLR5 activity (Fig. 4). However, the C-terminal fusion STF2.HA1-2 (VN) only induced about 65% of IL-8 secretion compared to the level of the R3 construct. The reduced TLR5 activity together with the lessened antigenicity seen previously suggest that linear fusion of flagellin and HA1-2 (VN) antigen may have intramolecular interactions that interfere the functionality of both fusion partners. By comparison, STF2R3.HA1-2 (VN) appeared to have a better construction to present both flagellin and HA1-2 (VN) antigen. STF2R0.HA1-2 (VN) behaved even more poorly in this assay and was consistent with previous reports [36,37] indicates that at least a portion or the full-length D0 of flagellin contributes to the TLR5 interaction. To exclude the possibility of contaminating endotoxin in the IL-8 assay, the Limulus Amebocyte Lysate (LAL) assay was performed and the endotoxin level was determined to be less than 0.02 EU/μg.
Fig. 4.
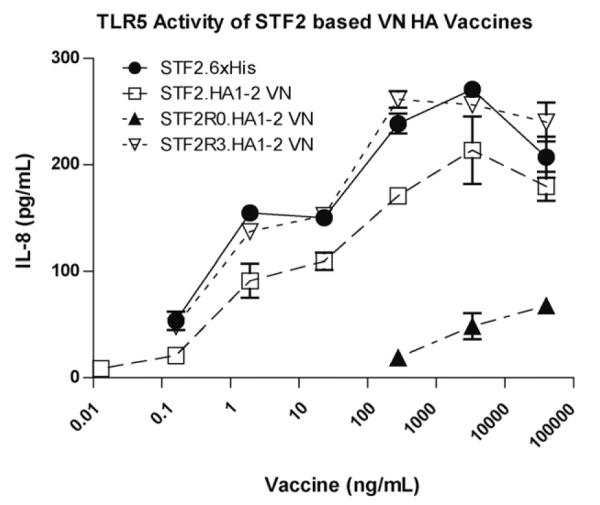
Comparative TLR5 activity of VN vaccine constructs. Each of the VN HA vaccines was tested for TLR5 activity using HEK-293 cells. Vaccines were compared to flagellin alone (STF2.6xHis) at the indicated doses ranging from 40,000 to 0.01 ng/ml. HEK-293 cells were incubated with vaccine constructs for 20 h and supernatant was tested for IL-8 using a specific antibody pair (BD). Data is shown as the mean ± standard deviation of IL-8 (pg/ml).
3.5. Efficacy of the STF2.HA1-2 (VN) vaccine
In the first efficacy study carried out, groups of 15 BALB/c mice were immunized twice at a 2-week interval with 1, 3, or 10 μg of the STF2.HA1-2 (VN) vaccine delivered s.c. Two weeks post the booster dose mice were challenged with the VN04 virus. A statistically significant (logrank test, p < 0.0001), dose-dependent decrease in severe disease and death relative to the placebo control group was observed. The survival rates for the 1, 3 and 10 μg dose groups were 18%, 40% and 73%, respectively. As expected, all of the control animals (placebo) developed severe disease and subsequently succumbed to the challenge (Table 1, Study 1).
Table 1.
Summary of survival rates following 2 or 3 immunizations of STF2.HA1-2 VN.
| Vaccine construct (VN) |
No. mice | Dose (μg) | 2 immunization % survival (Study 1) |
3 immunization % survival (Study 2) |
|---|---|---|---|---|
| STF2.HA1-2 | 15 | 10 | 73 | 100 |
| STF2.HA1-2 | 15 | 3 | 40 | 93 |
| STF2.HA1-2 | 15 | 1 | 18 | 87 |
| Placebo | 30 | 0 | 0 | 0 |
The clear relationship between dose level and efficacy from this first study suggested that efficacy could be further enhanced with doses of STF2.HA1-2 (VN) greater than 10 μg. The potential for augmenting the protection by further increasing the vaccine dose level was therefore evaluated. However, dosages of up to 30 μg of STF2.HA1-2 (VN) did not seem to improve the overall survival (data not shown).
The potential for augmenting the protection was further evaluated using three immunizations of the vaccine (Table 1, Study 2). In this study, doses of 1, 3, and 10 μg of STF2.HA1-2 (VN) were delivered at 42, 28 and 14 days pre-challenge. Higher survival rates for all dose groups were observed with 87%, 93%, and 100% of the mice surviving the challenge. None of the animals in the placebo (mock vaccinated) control group survived the challenge and the median survival was 7 days.
Study 2 was repeated with the additional evaluation of viral titers in the organs of five randomly pre-selected animals per group. Again, high survival rates of 80%, 87%, and 93% were observed. The control group succumbed to the disease in average 6 days. (Table 2, Study 3).
Table 2.
Virus loads in mouse brain and lung in Study 3 six days post-challenge.
| Vaccine construct (VN) |
No. mice in challenge |
Dose (μg) | Percent survival |
No. randomly pre-selected mice |
Brain | Lung | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||||
| Average titer | SD | Undetectable |
Average titer | SD | Undetectable | |||||||
| Proportion | % | Proportion | % | |||||||||
| STF2.HA1-2 | 15 | 10 | 93 | 5 | 0.00E0 | 0.00E0 | 5/5 | 100 | 3.00E4 | 6.71E4 | 4/5 | 80 |
| STF2.HA1-2 | 15 | 3 | 87 | 5 | 0.00E0 | 0.00E0 | 5/5 | 100 | 2.00E4 | 4.47E4 | 4/5 | 80 |
| STF2.HA1-2 | 15 | 1 | 80 | 5 | 0.00E0 | 0.00E0 | 5/5 | 100 | 2.20E5 | 4.38E5 | 3/5 | 60 |
| Placebo | 30 | 0 | 0 | 5 | 8.10E4 | 6.50E0 | 0/5 | 0 | 6.43E7 | 6.43E7 | 0/5 | 0 |
In Study 3, dose of 1, 3, and 10 μg of STF2.HA1-2 (VN) were delivered at 42, 28 and 14 days pre-challenge. Organs were collected from randomly pre-selected animals (N=5) on day 6 post-challenge and evaluated for levels of infectious virus in brain and lung. The group averages with standard deviation are presented.
In mice, the broader tissue tropism for HPAI viruses has been shown to be associated with a polybasic cleavage site which allows the virus to be easily cleaved by proteases at extra-pulmonary sites and to specific amino acid substitutions in the PB2 protein [38,39]. Although the tissue tropism and pathogenesis of these viruses is not as well defined in humans, there are reports of systemic infection in humans [13]. It was therefore relevant to study the virus loads in vaccinated animals in both lung and brain tissue. In Study 3, organs were collected from randomly pre-selected animals (N = 5) on day +6 and evaluated for levels of infectious virus. Individual values were recorded and the group averages plus the standard deviation are presented in Table 2. A difference of at least five log10 in the average brain titer was measured between the vaccinated and placebo groups, irrespective of the vaccine dose (1, 3 or 10 μg). Virus was below the limit of detection (<1 × 104 TCID50/g of tissue) in the brains of all vaccinated animals, whereas for the placebo group, the average titer was 4.9 (±4.8) log10 TCID50/g. In the lungs, a titer difference of 2.2–3.2 log10 was detected; for vaccinated animals the average titer was between 4.3 and 5.3 (±4.7–5.6) log10 TCID50/g, whereas the placebo average was 7.6 (±7.8) log10 TCID50/g. Virus was undetectable in 60% of the lungs of those vaccinated with 1 μg and 80% of those vaccinated with 3 or 10 μg of STF2.HA1-2 (VN). In contrast, virus could be detected in 100% of the lungs and brains of the placebo animals. Based on the 3 μg dose group results the level of protection was comparable between the first and second 3-dose trials.
Thus, the STF2.HA1-2 (VN) vaccine, when used in a 3-dose regimen, provided significant protection, which was consistent, as demonstrated by survival rates of ≥80% in two independent studies and reduced the virus titer in the brains and lungs.
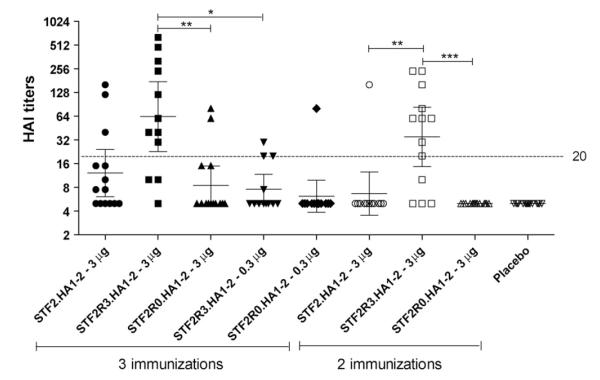
3.6. Immunogenicity and efficacy of R3 and R0 constructs
In a head-to-head efficacy study, doses of 3 or 0.3 μg of STF2.HA1-2 (VN), STF2R0.HA1-2 (VN) or STF2R3.HA1-2 (VN) were delivered either at days 42, 28 and 14 (2-week interval between doses) pre-challenge or days 42 and 21 (3-week interval between doses) pre-challenge. Serum samples were collected 12 days post the last boost, and subjected to a standard HAI test against A/Vietnam/1203/2004 (Fig. 5). The STF2R0.HA1-2 (VN) construct failed to elicit significant levels of serum HAI antibodies following either two or three immunizations. This is consistent with the low TLR5 activity of STF2R0.HA1-2 (VN) as shown in Fig. 4. STF2R3.HA1-2 (VN) elicited the highest HAI titers with GMTs of 63 and 35 following 3 and 2 immunizations of 3 μg, respectively. Significantly lower levels of HAI antibodies were elicited by 0.3 μg of STF2R3.HA1-2 (VN) (GMT = 8) as compared to 3 μg. With the two immunization regimen, STF2R3.HA1-2 (VN) was the only immunogen that induced significant levels of HAI antibodies. HAI titers of pooled STF2R3.HA1-2 (VN) samples were 160, 20, and 80 for 3 immunization of 3 and 0.3 μg, and 2 immunizations of 3 μg, respectively. STF2.HA1-2 (VN) induced intermediate levels of HAI antibodies.
Fig. 5.
Induction of serum HAI antibodies in mice immunized with various VN04 constructs. BALB/c mice were immunized either three times (3×) at 2-week intervals or twice (2×) at 3-week intervals. Mouse serum samples (N = 10–15) collected 12 days post 2nd or 3rd boost were treated with RDE, heat-inactivated, and subjected to HAI test with influenza A/Vietnam/1203/04 (H5N1) virus. The HAI titers were plotted individually with GMT (horizontal lines) and 95% CI (bars). Dashed line represents a 4-fold HAI titer of the baseline (5). Between the groups connected by bars: *p < 0.05, significant in Kruskal–Wallis/Dunns tests; **p < 0.01, very significant; ***p < 0.001, extremely significant.
Two (3 immunizations) or 3 weeks (2 immunizations) post the last booster dose, mice were challenged intranasally with ~10 × LD90 of the highly pathogenic A/Vietnam/1203/2004 strain. Survival and disease development were monitored for 20–21 days post-challenge (Table 3 and Fig. 6).
Table 3.
Survival rates after lethal challenge with H5 VN virus among mice immunized with 2 or 3 doses with 3 different HA VN globular head constructs fused to flagellin.
| Vaccine construct (VN) | No. mice | No. doses | Dose (μg) | Percent survival |
|---|---|---|---|---|
| STF2R3.HA1-2 | 10 | 2 | 3 | 100 |
| STF2R3.HA1-2 | 10 | 3 | 3 | 100 |
| STF2R3.HA1-2 | 10 | 3 | 0.3 | 80 |
| STF2.HA1-2 | 10 | 3 | 3 | 100 |
| STF2.HA1-2 | 10 | 3 | 0.3 | 60 |
| STF2.HA1-2 | 10 | 2 | 3 | 30 |
| STF2R0.HA1-2 | 10 | 3 | 0.3 | 10 |
| STF2R0.HA1-2 | 10 | 2 | 0.3 | 0 |
| STF2R0.HA1-2 | 10 | 3 | 3 | 0 |
| Placebo | 30 | 2 | 0 | 0 |
Fig. 6.
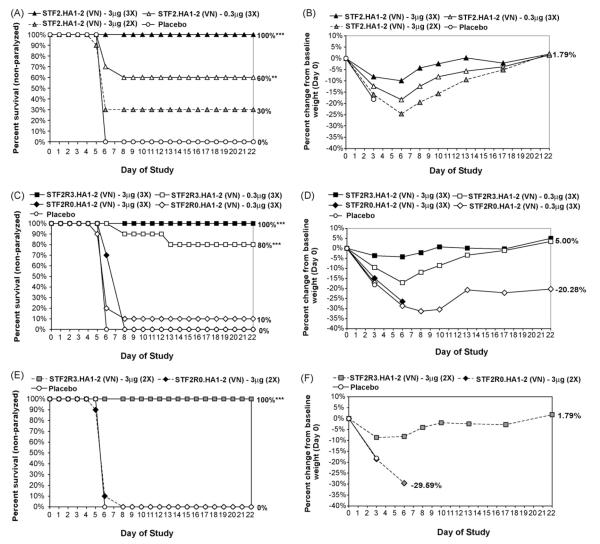
Efficacy for the different globular head constructs following a 3- or 2-dose regimen. Six-week-old female BALB/c mice were vaccinated s.c. with 3 or 2 immunizations of the indicated construct prior to challenge. On day 0, the mice were infected i.n. with influenza A/Vietnam/1203/2004 (VN04) with 6.8 × 104 TCID50/mouse. Clinical observations of disease development and mortality were monitored daily for 22 days. The percentage of survivors/non-paralyzed mice (A, C and E), and the percentage change in body weight from baseline on day 0 (B, D and F), are marked by group. Statistical analysis of survival comparing all groups over the post-challenge monitoring period was performed using logrank test at a significant level of α < 0.05. Logrank statistical significance is indicated by **p < 0.01, ***p < 0.0001. (A) plot of survival of the mice immunized two and three times with STF2.HA1-2 (VN); (B) percentage of body weight change of the mice immunized two and three times with STF2.HA1-2 (VN); (C) plot of survival of the mice immunized three times with STF2R3.HA1-2 (VN) and STF2R0.HA1-2 (VN); (D) percentage of body weight change of the mice immunized three times with STF2R3.HA1-2 (VN) and STF2R0.HA1-2 (VN); (E) plot of survival of the mice immunized two times with STF2R3.HA1-2 (VN) and STF2R0.HA1-2 (VN); (F) percentage of body weight change of the mice immunized two times with STF2R3.HA1-2 (VN) and STF2R0.HA1-2 (VN).
As expected, the alternative construct, STF2R0.HA1-2 (VN), was poorly efficacious with only 0–10% of the mice surviving in each of the different groups. This underscores the importance of a functional TLR ligand in driving a strong, protective immune response. None of the animals in the placebo (mock vaccinated) control group survived the challenge and the median survival was 6 days.
Similar to earlier results, 100% of animals receiving 3 doses of 3 μg of the STF2.HA1-2 (VN) vaccine survived the challenge, while only 30% of animals survived the challenge after 2 immunizations of 3 μg of the STF2.HA1-2 (VN) vaccine. In comparison, 100% of animals survived the challenge after receiving either 2 or 3 doses of 3 μg of the STF2R3.HA1-2 (VN) vaccine. Thus, the STF2R3.HA1-2 (VN) vaccine provides markedly improved efficacy against the highly pathogenic avian influenza virus challenge. Full survival and weight loss curves following the challenge are shown in Fig. 6.
In summary, replacement of domain D3 of flagellin with the VN04 HA globular head substantially improved the immunopotency and effectiveness of the vaccine relative to a C-terminal fusion of the HA globular head.
4. Discussion
We have evaluated several H5 subtype pandemic vaccines based on the influenza A/Vietnam/1203/2004 (VN04) virus. The vaccines all comprise the TLR5 ligand, flagellin, fused to the globular head domain of the VN04 HA and are efficiently manufactured using standard prokaryotic fermentation systems. We find that the R3 vaccine, in which domain D3 of flagellin is replaced with the HA globular head, is most effective at eliciting protective HAI titers and protecting mice from disease and death.
Based on earlier studies, our initial H5 vaccine design followed the same principles as a prototypic H1 vaccine which was shown to be highly protective in preclinical models [27]. In this original design, the HA globular head was fused to the C-terminus of the TLR5 ligand, flagellin. In the studies reported here, fusion of the VN04 globular head to the C-terminus of flagellin led to a vaccine that was protective in the mouse challenge model following three immunizations. However, in contrast to results with the H1 vaccine, when two immunizations of the VN04 C-terminal fusion vaccine were administered, some mice succumbed to the challenge. Although we observed dose effects on efficacy at the lower end of the dose response curve, increasing the dose above 10 μg did not further protect mice from disease for the 2-dose regimen. Full protection was only achieved by using three immunizations. Our interpretation of this result is that only a subset of the vaccine preparation consists of properly folded molecules and that a protective immune response could be selected and amplified by repeated exposure to this subset rather than the total mass of the vaccine. Thus, with the C-terminal fusion only a proportion of the molecules were effectively presenting the neutralizing epitopes located on the VN04 globular head and this in turn led to a requirement for multiple immunizations.
Structural and functional analyses of flagellin monomers have shown that domain D0, which comprises both the N- and C-terminal peptides, is unstructured and highly flexible when in solution. From a structural standpoint therefore, of the different construct designs, fusion of the HA globular head to the C-terminal peptide of domain D0 allows for the largest degree of molecular flexibility. This flexibility in structure could allow the VN04 HA globular head to interact intra-molecularly with flagellin, thereby shielding protective epitopes and possibly TLR5 binding site of flagellin. Alternatively, there could be an inherent instability specific to the VN04 head domain such that the flexibility of the C-terminal fusion is insufficient to stabilize the different antigenic regions. Consistent with both of these possibilities, we find that replacement of domain D3 of flagellin with the globular head domain substantially improved the antigenicity of the molecule as judged by reactivity with polyclonal and monoclonal antibodies. Replacement of domain D0 provided a modest improvement in antigenicity. The lack of efficacy associated with this vaccine is likely due to the loss of TLR5 activity. In contrast, domain D3 of flagellin is hypervariable and its removal does not interfere with TLR5 activity. In addition, domain D3 is structurally independent. Tethering both the N- and C-termini of HA globular head to this region of flagellin should at once limit the structural flexibility of the entire molecule as well as stabilize the VN04 HA globular head. Indeed, two doses of the R3 vaccine elicited protective HAI titers and provided 100% protection against disease and death.
Since the late-1990s outbreaks of highly pathogenic H5N1 avian influenza in the Asia-Pacific region have led to mounting concern that a pandemic threat is imminent. This has fueled an intense effort to develop protective vaccines that can be manufactured and distributed to meet global needs. Both conventional approaches, as well as unconventional techniques such as recombinant virus-like particles (VLPs) have been evaluated [32,40]. In one of the earliest efforts, conventional egg-based manufacturing was used to produce an inactivated, subvirion H5N1 vaccine based on a recombinant virus containing the HA and NA genes from influenza A/Vietnam/1203/2004 and the balance of genes from A/PR/8/34. In the Phase I clinical evaluation of this vaccine, 57% of the subjects were reported to seroconvert following two doses of 90 μg [41]. These early results highlighted the now widely recognized poor immunopotency associated with many of the H5 HA proteins and further intensified the need for more efficient production methodologies.
In subsequent studies, the use of adjuvants such as alum or oil–water emulsions to boost the potency of conventionally produced vaccines as well as multiple unconventional approaches to vaccine production have been evaluated. Studies in preclinical mouse models have demonstrated neutralizing titers generated in response to recombinant H5 HA from either insect or mammalian cells [42], protection against challenge using a DNA vaccine [43], as well as protection using VLPs from baculovirus [32,44]. Additionally, generation of significant HAI titers and protection has also been demonstrated in a ferret model using baculovirus rHA and VLPs [45]. The significance of adjuvants such as alum and oil–water emulsion has been shown in a macaque model [46]. While these results are promising, many of the barriers associated with potency and production remain unaddressed.
The intense focus on eukaryotic production systems stems from the historical view that protective forms of HA antigens must be manufactured using eukaryotic cells, like those of humans and chickens. We have now demonstrated that protective subunits of the H5 HA can be produced in bacterial expression systems. These HA subunits are recombinantly linked to flagellin to create an immunologically potent, protective vaccine that can be made quickly, inexpensively and in quantities sufficient to meet global needs. The efficiency of this technology translates approximately into a 1000-fold gain in capacity for influenza vaccine production [27].
In summary, we have developed an approach to the production of an efficacious pandemic influenza vaccine. The vaccine is comprised of the globular head domain of the protective hemagglutinin (HA) antigen fused to the potent TLR5 ligand, flagellin. The resulting fusion protein can be efficiently expressed in standard E. coli fermentation systems and the HA moiety can be faithfully refolded to take on the native conformation of the globular head. In mouse models of influenza infection, the vaccine elicits protective HAI titers that mitigate disease and protect mice from lethal challenge. These immunologically potent vaccines can be efficiently manufactured to support pandemic response and pre-pandemic vaccines.
Acknowledgments
We are grateful to Drs. Phil Wyde, Alan Shaw, Bob Becker and David Taylor for providing scientific input and technical training and to Jenna Linde for excellent data entry through the studies and for assistance in preparing the manuscript figures. We also thank Nathaniel Linde for technical assistance and Dr. Diana Noah and her team for performing the HAI assay at the BSL3 facility in Southern Research Institute. SP was supported by NIH K08 grant no. AI059491. Funding was provided by a grant from the Bill and Melinda Gates Foundation and VaxInnate Corporation.
References
- [1].Nobel GR. Basic and applied influenza research. CRC Press; Boca Raton, FL: 1982. Epidemiological and clinical aspects of influenza; pp. 11–50. [Google Scholar]
- [2].De Jong JC, Rimmelzwaan GF, Fouchier RA, Osterhaus AD. Influenza virus: a master of metamorphosis. J Infect. 2000 May;40(3):218–28. doi: 10.1053/jinf.2000.0652. [DOI] [PubMed] [Google Scholar]
- [3].Neumann G, Kawaoka Y. Host range restriction and pathogenicity in the context of influenza pandemic. Emerg Infect Dis. 2006 Jun;12(6):881–6. doi: 10.3201/eid1206.051336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Webster RG, Shortridge KF, Kawaoka Y. Influenza: interspecies transmission and emergence of new pandemics. FEMS Immunol Med Microbiol. 1997 Aug;18(4):275–9. doi: 10.1111/j.1574-695X.1997.tb01056.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Subbarao K, Katz J. Avian influenza viruses infecting humans. Cell Mol Life Sci. 2000 Nov;57(12):1770–84. doi: 10.1007/PL00000657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Sturm-Ramirez KM, Ellis T, Bousfield B, Bissett L, Dyrting K, Rehg JE, et al. Reemerging H5N1 influenza viruses in Hong Kong in 2002 are highly pathogenic to ducks. J Virol. 2004 May;78(9):4892–901. doi: 10.1128/JVI.78.9.4892-4901.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Ellis TM, Bousfield RB, Bissett LA, Dyrting KC, Luk GS, Tsim ST, et al. Investigation of outbreaks of highly pathogenic H5N1 avian influenza in waterfowl and wild birds in Hong Kong in late 2002. Avian Pathol. 2004 Oct;33(5):492–505. doi: 10.1080/03079450400003601. [DOI] [PubMed] [Google Scholar]
- [8].Shinya K, Hatta M, Yamada S, Takada A, Watanabe S, Halfmann P, et al. Characterization of a human H5N1 influenza A virus isolated in 2003. J Virol. 2005 Aug;79(15):9926–32. doi: 10.1128/JVI.79.15.9926-9932.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Normile D. Avian influenza. Wild birds only partly to blame in spreading H5N1. Science. 2006 Jun 9;312(5779):1451. doi: 10.1126/science.312.5779.1451. [DOI] [PubMed] [Google Scholar]
- [10].Cinatl J, Jr, Michaelis M, Doerr HW. The threat of avian influenza A (H5N1). Part I: epidemiologic concerns and virulence determinants. Med Microbiol Immunol. 2007 Mar 20;196(4):181–90. doi: 10.1007/s00430-007-0042-5. [DOI] [PubMed] [Google Scholar]
- [11].Poland GA, Jacobson RM, Targonski PV. Avian and pandemic influenza: an overview. Vaccine. 2007 Apr 25 20;(16):3057–61. doi: 10.1016/j.vaccine.2007.01.050. [DOI] [PubMed] [Google Scholar]
- [12].Lee VJ, Fernandez GG, Chen MI, Lye D, Leo YS. Influenza and the pandemic threat. Singapore Med J. 2006 Jun;47(6):463–70. [PubMed] [Google Scholar]
- [13].Beigel JH, Farrar J, Han AM, Hayden FG, Hyer R, de Jong MD, et al. Avian influenza A (H5N1) infection in humans. N Engl J Med. 2005 Sep 29;353(13):1374–85. doi: 10.1056/NEJMra052211. [DOI] [PubMed] [Google Scholar]
- [14].Epidemiology of WHO-confirmed human cases of avian influenza A(H5N1) infection. Wkly Epidemiol Rec. 2006 Jun 30;81(26):249–57. [PubMed] [Google Scholar]
- [15].WHO Epidemic and Pandemic Alert and Response (EPR): Cumulative Number of Confirmed Human Cases of Avian Influenza A/(H5N1) Reported to WHO. 2006 Aug 21; 2006 [cited; Available from: http://www. who.int/csr/disease/avian influenza/en/
- [16].Rowe T, Abernathy RA, Hu-Primmer J, Thompson WW, Lu X, Lim W, et al. Detection of antibody to avian influenza A (H5N1) virus in human serum by using a combination of serologic assays. J Clin Microbiol. 1999 Apr;37(4):937–43. doi: 10.1128/jcm.37.4.937-943.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Katz JM, Lim W, Bridges CB, Rowe T, Hu-Primmer J, Lu X, et al. Antibody response in individuals infected with avian influenza A (H5N1) viruses and detection of anti-H5 antibody among household and social contacts. J Infect Dis. 1999 Dec;180(6):1763–70. doi: 10.1086/315137. [DOI] [PubMed] [Google Scholar]
- [18].Cox MM. Cell-based protein vaccines for influenza. Curr Opin Mol Ther. 2005 Feb;7(1):24–9. [PubMed] [Google Scholar]
- [19].Hampson AW. Ferrets and the challenges of H5N1 vaccine formulation. J Infect Dis. 2006 Jul 15;194(2):143–5. doi: 10.1086/505229. [DOI] [PubMed] [Google Scholar]
- [20].Gerdil C. The annual production cycle for influenza vaccine. Vaccine. 2003 May 1;21(16):1776–9. doi: 10.1016/s0264-410x(03)00071-9. [DOI] [PubMed] [Google Scholar]
- [21].Akira S, Takeda K, Kaisho T. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat Immunol. 2001 Aug 8;2:675–80. doi: 10.1038/90609. [DOI] [PubMed] [Google Scholar]
- [22].Takeda K, Kaisho T, Akira S. Toll-like receptors. Annu Rev Immunol. 2003;21:335–76. doi: 10.1146/annurev.immunol.21.120601.141126. [DOI] [PubMed] [Google Scholar]
- [23].Ben-Yedidia T, Arnon R. Epitope-based vaccine against influenza. Expert Rev Vaccin. 2007 Dec 6;6:939–48. doi: 10.1586/14760584.6.6.939. [DOI] [PubMed] [Google Scholar]
- [24].Huleatt JW, Jacobs AR, Tang J, Desai P, Kopp EB, Huang Y, et al. Vaccination with recombinant fusion proteins incorporating Toll-like receptor ligands induces rapid cellular and humoral immunity. Vaccine. 2007 Jan 8;25(4):763–75. doi: 10.1016/j.vaccine.2006.08.013. [DOI] [PubMed] [Google Scholar]
- [25].McDonald WF, Huleatt JW, Foellmer HG, Hewitt D, Tang J, Desai P, et al. A West Nile virus recombinant protein vaccine that coactivates innate and adaptive immunity. J Infect Dis. 2007 Jun 1;195(11):1607–17. doi: 10.1086/517613. [DOI] [PubMed] [Google Scholar]
- [26].Huleatt JW, Nakaar V, Desai P, Huang Y, Hewitt D, Jacobs A, et al. Potent immunogenicity and efficacy of a universal influenza vaccine candidate comprising a recombinant fusion protein linking influenza M2e to the TLR5 ligand flagellin. Vaccine. 2008 Jan 10;26(2):201–14. doi: 10.1016/j.vaccine.2007.10.062. [DOI] [PubMed] [Google Scholar]
- [27].Song L, Nakaar V, Kavita U, Price A, Huleatt J, Tang J, et al. Efficacious recombinant influenza vaccines produced by high yield bacterial expression: a solution to global pandemic and seasonal needs. PLoS ONE. 2008;3(5):e2257. doi: 10.1371/journal.pone.0002257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].WHO . WHO manual on animal influenza diagnosis and surveillance. WHO; Geneva, Switzerland: 2002. [Google Scholar]
- [29].Stevens J, Corper AL, Basler CF, Taubenberger JK, Palese P, Wilson IA. Structure of the uncleaved human H1 hemagglutinin from the extinct 1918 influenza virus. Science. 2004 Mar 19;303(5665):1866–70. doi: 10.1126/science.1093373. [DOI] [PubMed] [Google Scholar]
- [30].Kendirgi F, Yun NE, Linde NS, Zacks MA, Smith JN, Smith JK, et al. Novel linear DNA vaccines induce protective immune responses against lethal infection with influenza virus type A/H5N1. Hum Vaccin. 2008 Nov-Dec;4(6):410–9. doi: 10.4161/hv.4.6.6177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Yun NE, Linde NS, Zacks MA, Barr IG, Hurt AC, Smith JN, et al. Injectable peramivir mitigates disease and promotes survival in ferrets and mice infected with the highly virulent influenza virus, A/Vietnam/1203/04 (H5N1) Virology. 2008 Apr 25;374(1):198–209. doi: 10.1016/j.virol.2007.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Bright RA, Carter DM, Crevar CJ, Toapanta FR, Steckbeck JD, Cole KS, et al. Cross-clade protective immune responses to influenza viruses with H5N1 HA and NA elicited by an influenza virus-like particle. PLoS ONE. 2008;3(1):e1501. doi: 10.1371/journal.pone.0001501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Ben-Yedidia T, Arnon R. Towards an epitope-based human vaccine for influenza. Hum Vaccin. 2005 May-Jun;1(3):95–101. doi: 10.4161/hv.1.3.1851. [DOI] [PubMed] [Google Scholar]
- [34].Yonekura K, Maki-Yonekura S, Namba K. Complete atomic model of the bacterial flagellar filament by electron cryomicroscopy. Nature. 2003 Aug 7;424(6949):643–50. doi: 10.1038/nature01830. [DOI] [PubMed] [Google Scholar]
- [35].Vonderviszt F, Kanto S, Aizawa S, Namba K. Terminal regions of flagellin are disordered in solution. J Mol Biol. 1989 Sep 5;209(1):127–33. doi: 10.1016/0022-2836(89)90176-9. [DOI] [PubMed] [Google Scholar]
- [36].Eaves-Pyles TD, Wong HR, Odoms K, Pyles RB. Salmonella flagellin-dependent proinflammatory responses are localized to the conserved amino and carboxyl regions of the protein. J Immunol. 2001 Dec 15;167(12):7009–16. doi: 10.4049/jimmunol.167.12.7009. [DOI] [PubMed] [Google Scholar]
- [37].Murthy KG, Deb A, Goonesekera S, Szabo C, Salzman AL. Identification of conserved domains in Salmonella muenchen flagellin that are essential for its ability to activate TLR5 and to induce an inflammatory response in vitro. J Biol Chem. 2004 Feb 13;279(7):5667–75. doi: 10.1074/jbc.M307759200. [DOI] [PubMed] [Google Scholar]
- [38].Hatta M, Gao P, Halfmann P, Kawaoka Y. Molecular basis for high virulence of Hong Kong H5N1 influenza A viruses. Science (New York, NY) 2001 Sep 7;293(5536):1840–2. doi: 10.1126/science.1062882. [DOI] [PubMed] [Google Scholar]
- [39].Katz JM, Lu X, Tumpey TM, Smith CB, Shaw MW, Subbarao K. Molecular correlates of influenza A H5N1 virus pathogenesis in mice. J Virol. 2000 Nov;74(22):10807–10. doi: 10.1128/jvi.74.22.10807-10810.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Treanor JJ, Wilkinson BE, Masseoud F, Hu-Primmer J, Battaglia R, O’Brien D, et al. Safety and immunogenicity of a recombinant hemagglutinin vaccine for H5 influenza in humans. Vaccine. 2001 Feb 8;19(13–14):1732–7. doi: 10.1016/s0264-410x(00)00395-9. [DOI] [PubMed] [Google Scholar]
- [41].Treanor JJ, Campbell JD, Zangwill KM, Rowe T, Wolff M. Safety and immunogenicity of an inactivated subvirion influenza A (H5N1) vaccine. N Engl J Med. 2006 Mar 30;354(13):1343–51. doi: 10.1056/NEJMoa055778. [DOI] [PubMed] [Google Scholar]
- [42].Wei CJ, Xu L, Kong WP, Shi W, Canis K, Stevens J, et al. Comparative efficacy of neutralizing antibodies elicited by recombinant hemagglutinin proteins from avian H5N1 influenza virus. J Virol. 2008 Jul;82(130):6200–8. doi: 10.1128/JVI.00187-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Bright RA, Ross TM, Subbarao K, Robinson HL, Katz JM. Impact of glycosylation on the immunogenicity of a DNA-based influenza H5 HA vaccine. Virology. 2003 Apr 10;308(2):270–8. doi: 10.1016/s0042-6822(03)00008-4. [DOI] [PubMed] [Google Scholar]
- [44].Crevar CJ, Ross TM. Elicitation of protective immune responses using a bivalent H5N1 VLP vaccine. Virol J. 2008;5:131. doi: 10.1186/1743-422X-5-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Mahmood K, Bright RA, Mytle N, Carter DM, Crevar CJ, Achenbach JE, et al. H5N1 VLP vaccine induced protection in ferrets against lethal challenge with highly pathogenic H5N1 influenza viruses. Vaccine. 2008 Oct 3;26(42):5393–9. doi: 10.1016/j.vaccine.2008.07.084. [DOI] [PubMed] [Google Scholar]
- [46].Ruat C, Caillet C, Bidaut A, Simon J, Osterhaus AD. Vaccination of macaques with adjuvanted formalin-inactivated influenza A virus (H5N1) vaccines: protection against H5N1 challenge without disease enhancement. J Virol. 2008 Mar;82(5):2565–9. doi: 10.1128/JVI.01928-07. [DOI] [PMC free article] [PubMed] [Google Scholar]