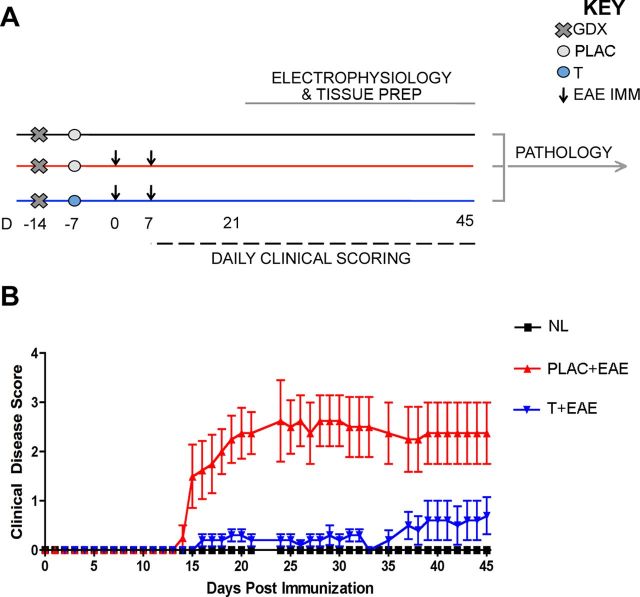
Figure 1.
Experimental design and standard clinical scores in EAE. A, Experimental design depicting the timing of gonadectomy (Day −14, denoted by x), placebo or testosterone pellet implantation (day −7, denoted by circles), EAE induction (day 0 and 7, denoted by arrows), daily clinical scoring (day 7–45, denoted by dashed line), and electrophysiology (day 21–45, denoted by gray line). Pertussis injections (intraperitoneal, i.p.) were given to both EAE groups on day 0 and 2, as part of standard EAE induction (data not shown). All mice were age-matched C57BL/6 adult males that were selected for electrophysiological recording in an intermixed fashion (one per day), so that mice from each experimental group were measured every 3 d. Brain tissue was separated depending on type of study: right hemispheres were used for electrophysiology; respective left hemispheres were prepared for pathology studies. All pathology experiments were conducted once all left hemisphere tissue from mice in all conditions had been collected. B, EAE clinical scores were recorded in placebo-treated (PLAC + EAE, red) or testosterone-treated (T + EAE, blue) mice as well as in non-EAE-induced healthy controls (NL, black). Placebo-treated mice with EAE exhibited a moderately severe clinical course, while testosterone-treated mice exhibited significantly reduced clinical severity. It is important to note that statistical variance was not altered as mice were individually selected for electrophysiological recording. Data are representative of two separate experiments. Repeated-measures ANOVA with post hoc pairwise comparisons revealed that PLAC + EAE was significantly different from other two groups, p < 0.05, n = 5 mice per group.