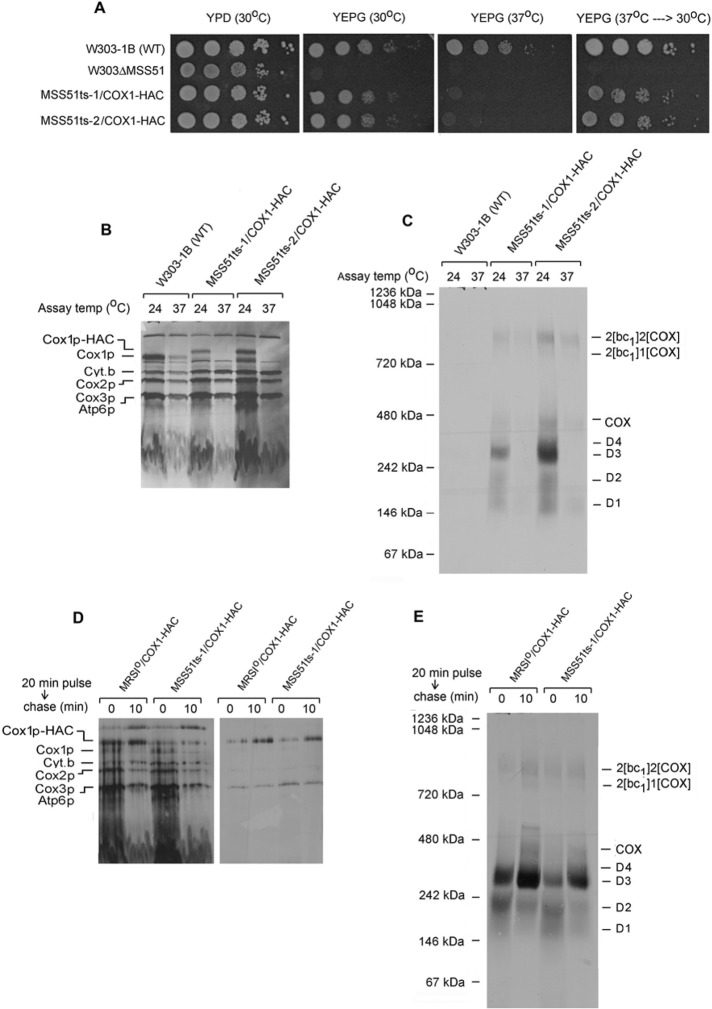
FIGURE 6:
Cox1p intermediates in an mss51 ts mutant. (A) Serial dilution of the wild-type W303-1B, the mss51-null mutant (W303ΔMSS51), and two independent mss51 ts mutants, MSS51ts-1/COX1-HAC and MSS51ts-2/COX1-HAC, were spotted on YPD and YEPG. The plates were incubated for 2 d at the indicated temperatures. The YEPG plate that had been incubated at 37°C was transferred to 30°C and incubated for an additional 2 d (far right). (B, C) Mitochondria were prepared from the wild-type strain W303-1B and the two mss51 ts mutants grown at 30°C. They were preincubated either at 24 or 37°C for 5 min before addition of [35S]methionine and cysteine and further incubated at the same temperatures for 30 min. Mitochondria were extracted with 1.2 volumes of 4% digitonin and the extracts purified on protein C antibody beads as in Figure 1A. The extracts were separated by SDS–PAGE on a 17% polyacrylamide gel (B) and the eluates from the beads by BN-PAGE (C). Proteins were transferred either to nitrocellulose or a PVDF membrane and exposed to x-ray film. (D) Mitochondria from the wild-type strain MRSIo/COX1-HAC and from the mss51 ts mutant were labeled for 20 min at 24°C with [35S]methionine and cysteine. Puromycin was added, and incubation continued for another 10 min at 37°C before extraction with digitonin. The extracts were purified on antibody beads, and samples of extract and eluates were separated by SDS–PAGE on a 17% polyacrylamide gel. Proteins were transferred to nitrocellulose and exposed to x-ray film. (E) The eluates from D were separated on a blue native gel, transferred to a PVDF membrane, and exposed to x-ray film.