Abstract
The lymphatic vascular system has an important role in the regulation of tissue pressure, immune surveillance and the absorption of dietary fat in the intestine. There is growing evidence that the lymphatic system also contributes to a number of diseases, such as lymphedema, cancer metastasis and different inflammatory disorders. The discovery of various molecular markers allowing the distinction of blood and lymphatic vessels, together with the availability of a increasing number of in vitro and in vivo models to study various aspects of lymphatic biology, has enabled tremendous progress in research into the development and function of the lymphatic system. This review discusses recent advances in our understanding of the embryonic development of the lymphatic vasculature, the molecular mechanisms mediating lymphangiogenesis in the adult, the role of lymphangiogenesis in chronic inflammation and lymphatic cancer metastasis, and the emerging importance of the lymphatic vasculature as a therapeutic target.
Introduction
Since its initial description back in the seventeenth century,1 the lymphatic system has probably never received as much scientific attention as during the last decade. Being the second vascular system found in higher vertebrates in addition to the blood vasculature, it has several vital functions including the regulation of tissue pressure, immune surveillance and the absorption of dietary fat in the intestine. Interest in basic lymphatic research was boosted by the growing evidence that the lymphatic system also contributes to a number of diseases, such as lymphedema, cancer metastasis and different inflammatory disorders. The discovery of various molecular markers allowing the distinction of blood and lymphatic vessels, together with the availability of a increasing number of in vitro and in vivo models to study various aspects of lymphatic biology, has enabled tremendous progress in our understanding of the development and function of the lymphatic system.
Lymphatic capillaries start blind-ended in the tissue, where they take up lymph, a protein-rich exudate from blood vessels. They are lined by a single layer of overlapping endothelial cells and lack a continous basement membrane as well as pericyte or smooth muscle cell coverage. Tissue fluid likely enters these intitial lymphatic vessels in between discontinous button-like cell junctions.2 Via larger collecting lymphatic vessels and ultimately the thoracic duct, it is returned to the blood vasculature through the lymphatico-venous connections at the junction of the jugular and subclavian veins. In the intestine, specialized lymphatic vessels, so-called lacteals, take up dietary fat and fat-soluble vitamins to transport them to the venous circulation. Unlike the blood vascular system, the lymphatic system does not feature a central pump, instead lymph is moved forward by skeletal muscle action, respiratory movement and contraction of smooth muscle in walls of collecting lymphatic vessels.
Besides the vessels, which are found in almost all tissues except for avascular structures such as epidermis, hair, nails, cartilage and cornea, and some vascularized organs including the brain and the retina, the lymphatic system also comprises the lymphoid organs. These include lymph nodes, thymus, tonsils, spleen and Peyer’s patches and are crucial for the immune function of the lymphatic system. Immune cells such as lymphocytes and antigen-presenting dendritic cells are transported via lymphatic vessels from the skin and other organs to regional lymph nodes, where specific immune responses are initiated.
Embryonic Development of the Lymphatic Vasculature
Two concepts for lymphatic development in the embryo are controversially discussed up to date, both of which have been proposed already at the beginning of the 20th century. The “centrifugal” model, presented in 1902 by Florence Sabin, proposes that primary lymph sacs arise from endothelial cells budding off the veins during early embryonic development. The peripheral lymphatic system is subsequently formed by endothelial sprouting from these lymph sacs into the surrounding tissues and organs.3 The “centripetal” model, introduced by Huntington and McClure in 1908, alternatively suggests the existence of mesenchymal precursor cells, so-called lymphangioblasts, from which the lymph sacs originate independently of the veins.4
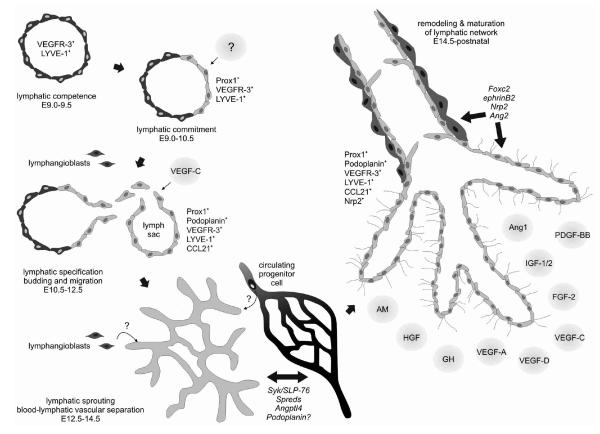
Support for Sabin’s centrifugal model was provided by studies in Prox1 deficient mice.5, 6 Prox1 is a homolog of the Drosophila homeobox transcription factor prospero 7 and acts as a master regulator of lymphatic development. Induced by a yet unknown signal, it becomes expressed in a subset of endothelial cells on one side of the cardinal vein around mouse embryonic day (E) 9.5–10.5 (Fig. 1). These lymphatically “competent” cells subsequently become lymphatically committed and specified, and eventually give rise to the lymphatic vasculature throughout the body. Prox1 null mice completely lack a lymphatic vascular system, since the budding and sprouting of lymphatically specified endothelial cells from the embryonic veins halts prematurely at around E 11.5–12.0 6 (Table 1). Corroborating this evidence, the venous origin of the mammalian lymphatic vasculature has recently been demonstrated by lineage-tracing experiments 8 and is further supported by studies in zebrafish, in which lymphatic endothelial cells (LECs) of the thoracic duct arise from primitive veins.9 In avians and Xenopus frogs, however, parts of the lymphatic system are derived from adjacent veins while others likely originate from local lymphangioblasts, which provides support for the centripetal model of lymphatic development.10–12 Whether and how lymphangioblasts might also contribute to embryonic lymphangiogenesis in mammals remains unknown. Evidence for the existence of lymphangioblasts also in mammals comes from murine embryoid bodies. In these three-dimensional, embryonic stem cell-derived structures LECs seem to not only bud off pre-existing blood vessel-like structures, but were also found away from vascular areas, where they might either have migrated to, or developed locally from lymphatic progenitors.13,14 Furthermore, scattered mesenchymal cells expressing the leucocyte marker CD45, the pan-endothelial marker CD31 and the lymphatic endothelial markers Prox1 and LYVE-1 were recently observed in mouse embryos and suggested to be lymphendothelial precursors capable of integrating into growing lymphatics.15
FIG. 1.
Lymphatic vasculature development and growth. During early embryonic development, all endothelial cells of the cardinal vein display lymphatic competence and express the lymphatic markers LYVE-1 and VEGFR-3. Induction of Prox1 expression in a subset of endothelial cells on one side of the vein marks commitment to the lymphatic lineage. Subsequent budding and migration of these cells to form the primary lymph sacs depends on VEGF-C signals. Several genes are required for the sustained separation of the developing lymphatic and blood vasculature, while others become important for the remodeling of the initial lymphatic network into capillaries and collecting vessels. During these processes, lymphatic endothelial cells adopt the expression of additional lineage markers. The relevance of lymphangioblasts and circulating progenitor cells for mammalian lymphatic development is unclear. Postnatally, lymphatic vessel growth is promoted by numerous factors. AM, adrenomedullin; Ang, angiopoietin; Angptl, angiopoietin-like protein; E, mouse embryonic day; FGF, fibroblast growth factor; GH, growth hormone; HGF, hepatocyte growth factor; IGF, insulin-like growth factor; Nrp2, neuropilin-2; PDGF, platelet-derived growth factor; VEGF, vascular endothelial growth factor.
Table 1.
Genetic Mouse Models Displaying Lymphatic Phenotypes
| Genes | Function | Models | Phenotype |
|---|---|---|---|
| Prox16,101 | Transcription factor |
KO | No lymphatic vasculature (−/−), adult-onset obesity, chylous ascites (+/−) |
| VEGF-C16 | Growth factor | KO | No lymphatic vasculature (−/−), delayed lymphatic vascular development, lymphatic hypoplasia and lymphedema (+/−) |
| VEGFR-319 | Growth factor receptor |
KO | Cardiovascular failure, defective remodelling of vascular networks |
| VEGFR-392 | Growth factor receptor |
Chy mice (inactivating mutation) |
Lymphedema, chylous ascites, hypoplastic cutaneous lymphatic vessels |
| LYVE-122,23 | Hyaluronan receptor |
KO | None or only subtle defects: increased interstitial- lymphatic flow, atypical shape of vessel lumen |
| Syk/SLP-7624,25 | Tyrosine kinase/ adaptor protein |
KO | Abnormal blood-lymphatic connections, chylous ascites, defect in hematopoietic endothelial progenitors |
| Spred-1/Spred-226 | Negative regulators of ERK activation |
Double KO | Lymphedema, dilated and blood filled lymphatic vessels |
| Angptl427 | Inhibitor of lipoprotein lipase |
KO | Postnatal lymphatic-venous partitioning defect in the small intestine |
| Podoplanin37 | Membrane glycoprotein |
KO | Lymphedema, dilatation and mispatterning of lymphatic vessels, diminished lymphatic transport |
| Foxc228,139 | Transcription factor |
KO | Lymphatic hyperplasia, retrograde lymph flow (+/−), abnormal patterning and pericyte investment of lymphatic vessels, absence of valves, lymphatic dysfunction (−/−) |
| Foxc1/Foxc2140 | Transcription factors |
Compound mutant (Foxc1 +/−; Foxc2 −/−) |
Defective lymphatic sprouting from veins during development (reduced VEGF-C expression) |
| Ephrin B232 | Ligand of EphB receptors |
Mutant lacking PDZ interaction site |
Defective remodeling of lymphatic vascular network, hyperplasia, lack of valves, chylothorax |
| Neuropilin-231 | Growth factor receptor |
KO | Transient absence or severe reduction of small lymphatic vessels and capillaries during development |
| Angiopoietin-229,30 | Growth factor | KO | Chylous ascites and subcutaneous edema, abnormal patterning of lymphatic vessels, abnormal periendothelial cell coverage of lymphatic capillaries |
| Aspp1141 | p53-binding protein |
KO | Subcutaneous edema, disorganized and non-functional lymphatic vasculature in embryo, mispatterned collecting lymphatic vessels in adult |
| Andrenomedullin89 | Vasoactive peptide |
KO | Interstitial lymphedema (KO), abnormal jugular lymphatic vessels (conditional KO in ECs) |
| Emilin-1142 | ECM protein | KO | Hyperplastic, enlarged, irregularly patterned lymphatic vessels, reduction of anchoring filaments, lymphedema |
| Integrin α952 | Adhesion receptor | KO | Chylothorax, lymphedema |
| VEGF-C63 | Growth factor | TG (K14) | Hyperplastic lymphatic vessels |
| VEGF-D64 | Growth factor | TG (K14) | Hyperplastic lymphatic vessels |
| VEGF-A72 | Growth factor | TG (K14) | Enlarged lymphatic vessels |
| HGF80 | Growth factor | TG | Increased number and enlargement of lymphatic vessels |
| Angiopoietin-179 | Growth factor | TG (K14) | Lymphatic vessel enlargement and sprouting |
| Net (Elk3)143 | Transcription factor |
KO | Chylothorax, dilated lymphatic vessels |
| SOX18 (ragged)95 |
Transcription factor |
Spontaneous missense mutations |
Edema, chylous ascites, cardiovascular and hair follicle defects |
| Pi3kca144 | Phosphoinositide 3-kinase |
Mutant unable to interact with Ras |
Chylous ascites, reduction of lymphatic capillaries |
| Trisomy 16145 | various | Triplication of chromosome 16 |
Abnormal size and structure of jugular lymph sacs, nuchal edema |
Angptl4, angiopoietin-like protein 4; Aspp, apoptosis simulating protein of p53; ECM, extracellular matrix; Foxc, forkhead box C; HGF, hepatocyte growth factor; K14, keratin 14; KO, knockout; LYVE-1, lymphatic vascular endothelial hyaluronan receptor-1; PDZ, PSD-95, DISCS-large, and ZO-1; Pi3kca, phosphoinositide 3-kinase p110a; SLP, Src homology 2-domain containing leukocyte protein; SOX, sex determining region Y-related high mobility group box; Spred, Sprouty-related Ena/VASP homology 1 domain-containing protein; TG, transgenic; VEGF, vascular endothelial growth factor; VEGFR, vascular endothelial growth factor receptor.
Studies of knockout mouse models have revealed a number of genes involved in the embryonic development of the lymphatic system, in addition to Prox1 (Table 1). Among those, vascular endothelial growth factor (VEGF)-C and— most likely—its receptor VEGFR-3 are indispensable for early lymphatic development as revealed by deletion of VEGF-C in mice, resulting in a complete lack of lymphatic vasculature and prenatal death due to fluid accumulation in the tissues.16 The VEGF-C signal during embryonic development is required for the budding and sprouting of the Prox1-positive lymphatically committed endothelial cells from the veins to form the initial lymph sacs (Fig. 1). The lack of VEGF-D, however—the only other known ligand of VEGFR-3—does not affect lymphatic development.16,17 Exogenous, but not endogenous, VEGF-D can rescue the phenotype of VEGF-C deficient mice, suggesting that VEGF-D is not expressed at the critical sites of lymph sac formation in the embryo.16,18 Deletion of VEGFR-3 causes embryonic death due to cardiovascular failure at E 9.5, before the initiation of lymphatic development,19 which demonstrates its necessity for blood vascular development but precludes the assessment of its role in lymphatic development. While in normal adult tissues VEGFR-3 expression is largely restricted to lymphatic endothelium, at this time point in early embryonic development VEGFR-3 is present on both venous and presumptive lymphatic endothelia20 (Fig. 1). In fact, the earliest marker of lymphatic competence is lymphatic vascular endothelial hyaluronan receptor-1 (LYVE-1), a homolog of the blood vascular endothelium-specific hyaluronan receptor CD44.21 It is expressed on endothelial cells of the anterior cardinal vein as early as E 9.0–9.5 (Fig. 1) and is one of the most widely exploited markers for lymphatic endothelium. Nevertheless, LYVE-1 knockout mice have no or only subtle lymphatic defects,22,23 and the biological function of this protein is yet unknown.
Once the lymph sacs are formed, the blood and the lymphatic vascular systems continue to develop separately. Only few connections are maintained to allow the return of lymph to the blood circulation. Several molecules were found to be involved in controlling the separation of the two types of vasculature (Fig. 1), including the tyrosine kinase Syk and the adaptor protein SLP-76. Inactivation of either of these genes in mice results in abnormal blood-lymphatic connections during embryogenesis 24 (Table 1). Since Syk and SLP-76 expression is restricted mainly to hematopoietic cells and not detectable in endothelial cells, intact Syk- and SLP-76-signaling in hematopoietic cells—which might be a potential source of endothelial precursors during development—appears to be required to prevent blood-lymphatic vascular mixing.24, 25 A similar phenotype, featuring blood-filled lymphatic vessels in the embryo, was reported for Spred-1/Spred-2 double knockout mice. Spreds suppress VEGF-C signaling by inhibiting VEGFR-3 mediated ERK activation,26 suggesting a role for VEGFR-3 signaling in vascular separation. A recent study in angiopoietin-like protein 4 (Angptl4, also known as fasting-induced adipose factor, Fiaf) knock-out mice moreover indicates that active mechanisms are required for sustained separation of blood and lymphatic vessels postnatally.27 Within a few days after birth, these animals develop lethal defects in the partitioning of the lymphatic and blood microvasculature in the intestine, associated with reduced Prox1 expression in the LECs of the small intestine.
The final steps of lymphatic development—the remodelling and maturation of the initial lymphatic vessel network into lymphatic capillaries and collecting lymphatic vessels— require the sprouting of new lymphatic capillaries from pre-existing ones, as well as the aquisition of mural cell coverage around and the formation of valves within collecting lymphatic vessels (Fig. 1). The transcription factor Foxc2,28 the growth factor angiopoietin-2,29, 30 the non-kinase receptor neuropilin-2,31 and the Eph receptor ligand ephrinB232 have been identified as molecular mediators of these processes based on knockout mice exhibiting corresponding lymphatic phenotypes at late stages of embryonic development (Table 1). Also the transmembrane glycoprotein podoplanin, which is expressed on LECs but not blood vascular endothelial cells (BECs) in vivo and in vitro and is an established marker for lymphatic endothelium,33–37 appears to be required for correct formation and function of the mature lymphatic vasculature. Podoplanin knockout mice die at birth due to respiratory failure and in addition exhibit various lymphatic defects37,38 (Table 1). Podoplanin is capable of aggregating platelets through interaction with the C-typelectin-like receptor 2 (CLEC-2) on platelets,39,40 and is involved in the cytoskeletal organization of endothelial and other cells.37,41,42 Its exact molecular function, however, has remained unknown up to date. Given the phenotype of the Syk/SLP-76 knockout mice 24 together with the fact that CLEC-2 signals via Syk and SLP-76 to aggregate platelets,43 one might speculate on a potential function of podoplanin in preventing anastomoses between lymphatic and blood vessels by thrombus formation.
Although the endothelial cells lining lymphatic vessels originate from blood vascular endothelial cells and hence these two cells types are genetically closely related, in the course of development LECs increasingly acquire specific expression of diverse genes, distinguishing them from BECs and reflecting the distinct functions of the two vascular systems. In addition to the above mentioned markers Prox1, VEGFR-3, LYVE-1 and podoplanin, these genes include for instance the lymphatic-specific chemokine CCL2144 and neuropilin-2,31 a co-receptor for several growth factors including VEGF-C 45 and hepatocyte growth factor (HGF).46 Thanks to the possibility of isolating LECs and BECs from human skin and propagating them in culture for several passages without loss of their lineage-specific differentiation,34–36,47 the molecular differences between the two cell types could be explored in great detail on the transciptome34,36 as well as on the proteome level.48 In addition, recent studies comparing freshly isolated and cultured LECs have highlighted the considerable impact of the in vivo tissue microenvironment on the trancriptional profiles of endothelial cells.49,50
A crucial role in the lineage-specification of LECs versus BECs must be assigned to Prox1. Its ectopic expression in BECs induces the expression of lymphatic marker genes in these cells,36,51 whereas the acquisition of lymphatic marker expression by budding endothelial cells during embryonic development is abolished in Prox1 null mice.5 Recently, integrin α9—which was shown to be involved in the development of the lymphatic system 52 (Table 1) and to be a receptor for VEGF-C and -D53—and VEGFR-3 have been identified as target genes of Prox1,54 contributing to LEC migration towards VEGF-C signals. Corroborative for this potential novel role of Prox1 in cell migration, we recently found that it promotes the invasiveness of kaposiform hemangioendothelioma.55
Lymphangiogenesis in the Adult
In adult organisms, lymphangiogenesis takes place only in certain pathological conditions such as tissue repair, inflammation and tumor growth. At present, it is unclear whether lymphatic vessel growth in these settings is exclusively due to proliferation of local endothelial cells and sprouting of pre-existing vessels, or whether it involves also the incorporation of circulating endothelial progenitor cells at sites of active lymphangiogenesis.
Putative lymphatic endothelial progenitor cells, co-expressing lymphatic endothelial and stem cell markers, have been identified in human fetal liver and cord blood.56 Nevertheless, experiments using sublethally irradiated mice grafted with GFP-expressing bone marrow suggested that bone marrow-derived endothelial progenitor cells do not contribute to tumor- or VEGF-C-induced lymphangiogenesis, since no GFP-positive donor cells were observed in the newly formed lymphatic vessels when growth of lymphatic vasculature was induced by VEGF-C application or by tumor implants in these mice.57 In contrast to this, bone marrow-derived cells were incorporated into growing lymphatic vessels in the inflamed or fibroblast growth factor (FGF)-2 treated corneas of GFP chimeric mice,58,59 as well as during inflammation-associated lymphangiogenesis in human renal transplants.60 These cells have been suggested to be macrophages, which transdifferentiate into LECs,59,60 and their relative contribution to the new vessels appears to be minor. Macrophages and other inflammatory cells may, however, in addition play an indirect role in neovascularization through the secretion of lymphangiogenic factors such as VEGF-C or VEGF-D.61,62
VEGF-C and the structurally closely related VEGF-D are the most important and best characterized lymphangiogenic growth factors to date. When overexpressed in the skin of mice, they induce hyperplasia of cutaneous lymphatic vessels63,64 (Table 1)—in contrast to VEGF-C, VEGF-D does so mainly postnatally rather than in the embryo.65 VEGF-C moreover promotes the growth, migration and survival of cultured human LECs.47 The principal receptor of VEGF-C and -D is VEGFR-3; after proteolytic cleavage, however, these growth factors can also bind VEGFR-2.66–69 While it has been shown that exclusive activation of VEGFR-3 signaling is sufficient to promote lymphangiogenesis,64 the contribution of VEGFR-2 signals to lymphangiogenesis is less clear. In this context, several studies have demonstrated the lymphangiogenic potential of VEGF-A—which is a ligand of VEGFR-2 but not VEGFR-3—in vitro 34 and in vivo.70–73 Although VEGF-A might act indirectly by attracting VEGF-C and -D-producing inflammatory cells,61,62,74 at least part of its effects on lymphatic vessels can be attributed to VEGFR-2, since they can be abolished by antibodies specifically blocking this receptor.71,72 A recent study, dissecting VEGFR-3 independent mechanisms of lymphangiogenesis, suggests that VEGFR-2 signals in lymphatic vessels promote their enlargement but not the formation of new vessel sprouts.75 In an adult model of lymphangiogenesis in regenerating skin, both VEGFR-2 and VEGFR-3 were required for LEC migration and proliferation, while signaling via either of them alone appeared to be sufficient for the subsequent organization of LECs into functional capillaries.76 The question about a possible temporal component of VEGFR-3 dependent versus independent lymphangiogenesis was recently addressed by application of a soluble VEGFR-3-Ig fusion protein in postnatal and adult mice to block signaling through VEGFR-3. This study suggests that, postnatally, VEGF-C/D signals via VEGFR-3 are only required for the maintenance of small lymphatic vessels during the first few weeks of life. Thereafter, lymphatic vessels regenerate in spite of constant VEGFR-3 inhibition.65 In agreement with these findings, blockade of VEGFR-3 in adult mice by means of a neutralizing antibody specifically inhibits the growth of new lymphatics, leaving the survival and function of pre-existing vessels unaffected.77 Altogether, this indicates that other factors might become important for the growth and maintenance of lymphatic vessels during adult life. Indeed, a multitude of growth factors capable of promoting lymphangiogenesis have been identified up to date, including angiopoietin (Ang)-1,78,79 hepatocyte growth factor (HGF),80, 81 fibroblast growth factor (FGF)-2,82–84 insulin-like growth factor (IGF) 1 and 2,85 and platelet derived growth factors (PDGF).86 Some of these may act on lymphatic vessels indirectly via the VEGF-C/VEGFR-3 signaling pathway or only in certain pathological situations (reviewed in87).
Newly identified lymphangiogenic factors include the growth hormone (GH),88 and the multifunctional peptide adrenomedullin (AM), which signals via the calcitonin receptor-like receptor (calcrl) associated with the receptor activity modifying protein (RAMP) 2. In addition to AM signaling having a role in lymphatic development as revealed by knockout mice for either AM itself or calcrl or RAMP2 (Table 1), adrenomedullin treatment promotes the proliferation of cultured human LECs.89
The Lymphatic System in Disease
Lymphedema
The principal physiological function of the lymphatic vasculature is to take up fluid, leaking out of blood capillaries into interstitial spaces in the tissue, and to return it to the blood circulation. Any failure to effectively do so results in lymphedema, a chronic, disabling and disfiguring condition. The accumulation of protein-rich fluid in the tissues causes swelling of the extremities and is in most patients also associated with inflammatory reactions, fibrosis, overgrowth of adipose and connective tissue in the affected areas, and other symptoms.
Based on its cause, lymphedema can be classified into primary (hereditary) and secondary (acquired) forms. Primary lymphedema is rare and generally characterized by hyper- or hypoplastic lymphatic vessels and/or insufficient function of lymphatic valves. A variety of distinct syndromes have been described, for some of which the underlying genetic defects could be identified. Milroy’s disease, a form of congenital lymphedema with autosomal dominant inheritance, can be ascribed to kinase-inactivating mutations in the VEGFR-3 gene, found in several affected families,90,91 as well as in Chy mice which have hypoplastic cutaneous lymphatic vessels associated with lymphedema92 (Table 1). Another hereditary form of lymphedema, with a later onset around puberty, is lymphedema-distichiasis syndrome—so called because, in addition to edema, patients often have double rows of eyelashes (distichiasis). This disorder is caused by dominant, inactivating mutations in the FOXC2 gene,93 which encodes a forkhead-related transcription factor required for the proper formation of lymphatic valves and the regulation of appropriate pericyte recruitment to lymphatic vessels28 (Table 1). Finally, mutations in the gene encoding SOX18, a SRY-related transcription factor, have been described to cause recessive and dominant forms of hypotrichosis-lymphedema-telangiectasia syndrome.94 SOX18 is known to be important for blood vascular development, and the phenotype of ragged mice, which have a spontaneous missense mutation in the Sox18 gene, indicates that SOX18 is also involved in lymphatic development95 (Table 1). In the majority of families with lymphedema, however, none of the above mentioned mutations occurs, thus several more genes relevant for lymphedema are still awaiting their discovery.
Secondary lymphedema develops upon obstruction of lymphatic vessels, which—in industrialized countries—is mostly the result of surgery or radiotherapy for breast cancer, whereas in tropical countries lymphatic filariasis, a mosquito-borne parasitic infection, is the most common cause of lymphedema (reviewed in 96).
Up to date, no curative treatment for lymphedema exists, and therapeutic measures are limited to manual lymph drainage and compression bandaging. Based on its potent lymphangiogenic effect, VEGF-C has been tested for its ability to improve lymphatic function in animal models of lymphedema. In Chy mice, virus-mediated VEGF-C gene therapy stimulated the growth of functional cutaneous lymphatic vessels,92 and surgically induced lymphedema in a rabbit ear model could successfully be treated by injection of recombinant VEGF-C protein.97 The unwanted growth and leakiness of blood vessels, resulting from activation of VEGFR-2 by VEGF-C, could be circumvented using a VEGFR-3-specific mutant form of VEGF-C.98 While these studies have demonstrated the reconstitution only of lymphatic capillaries, it has recently been shown that by adenoviral delivery of VEGF-C or -D also functional and mature collecting lymphatic vessels can be regenerated and connected to lymph node transplants within six months after surgical removal of the axillary lymph nodes and all of the associated collecting lymphatic vessels in adult mice.99
The lymphatic vasculature in lipid homeostasis
Given the role of lymphatic vessels in mediating the uptake of lipids from the intestine, the accumulation of fat observed in the edematous tissues of lymphedema patients, the close anatomical association of lymph nodes with adipose tissue, and the ability of lymph to promote the differentiation of pre-adipocytes to mature adipocytes in vitro,100,101 one might suspect a connection between lymphatic function and lipid homeostasis. A recent study in adult Prox1 +/− mice— which survive exclusively on the NMRI background and are obese compared to their wild-type littermates—indeed suggests a link between lymphatic dysfunction and adult-onset obesity. In these mice, abnormal leakage of lymph mainly from mesenteric lymphatic vessels appears to promote lipid accumulation in adipocytes as well as adipogenic differentiation.101
The role of lymphatic vessels in inflammation
Inflammation occurs as a response to tissue injury or infection, in the context of autoimmune diseases and during tumor growth. The biological role of lymphatic vessels in the pathogenesis of inflammation is not fully clarified up to date. On the one hand, they might drain inflammation-associated edema and participate in the removal of immune cells and inflammatory cytokines from the site of infection—on the other hand, they are crucial for the establishment of immune responses by serving as exit routes for activated antigen-presenting cells from the site of infection to regional lymph nodes. Increasing evidence suggests that lymphatic vessels might actively participate in the inflammatory process.
Lymphatic hyperplasia and/or enhanced lymphangiogenesis was observed in human psoriatic skin lesions,72 in the intestinal mucosa of ulcerative colitis patients,102 in the joints of mice with inflammatory arthritis,103 as well as during kidney transplant rejection104 and chronic airway inflammation in mice.74
VEGF-A appears to have a crucial role in inflammation-associated lymphangiogenesis. Its expression is increased in human psoriatic skin,105 and mice overexpressing VEGF-A in the epidermis display a prolonged inflammatory response after induction of cutaneous delayed-type hypersensitivity (DTH) reactions, which is associated with LEC proliferation and lymphatic hyperplasia and can be inhibited by blockade of VEGFR-1 and -2.72 In fact, lymphangiogenesis associated with chronic DTH reactions in mice was recently found to strongly depend on VEGF-A, as it could be prevented by means of a VEGF-A neutralizing antibody.106 Interestingly, in this study VEGF-A not only induced lymphangiogenesis at the site of inflammation where it was produced, but also in the draining lymph nodes. Enhanced lymph node lymphangiogenesis was also observed after immunization; in this case, however, it appeared to be mediated by B cells producing lymphangiogenic factors within the lymph node itself.107
VEGF-A-mediated inflammatory lymphangiogenesis might at least partially be the result of the recruitment of macrophages, supplying VEGF-C and –D.61,62 In addition, macrophages were reported to physically contribute to lymphangiogenesis by incorporation into newly forming lymphatic vessels in the inflamed mouse cornea.59
Our recent studies of lymphangiogenesis in UVB-irradiated mouse skin suggest that VEGF-A predominantly induces leaky, nonfunctional lymphatic vessels. Upon UVB-ir-radiation, an increase in VEGF-A expression goes along with the development of hyperpermeable, functionally impaired lymphatic vessels in the skin, and these vessel abnormalities could be prevented by administration of a neutralizing VEGF-A antibody.108 Overall, inflammation-associated lymphangiogenesis induced by VEGF-A appears to have a rather aggravating effect on the inflammatory process.
VEGF-C-mediated lymphangiogenesis, however, might have a more favorable role in inflammation. In inflamed tissues, VEGF-C is secreted predominantly by immune cells such as dendritic cells, macrophages and neutrophils, and its expression is upregulated in response to proinflammatory cytokines.74,109 High levels of VEGF-C were detected in arthritic joint synovium of rheumatoid arthritis patients.110 In joints of mice with inflammatory arthritis, VEGF-C—secreted by TNFα stimulated CD11b-positive cells—induced lymphangiogenesis,103 and enhanced lymphatic drainage appeared to have a beneficial effect on the resolution of inflammation in these mice.111,112 In agreement with these findings, inhibition of VEGFR-3 signaling by soluble VEGFR-3-Ig prevented lymphangiogenesis in a mouse model of chronic airway inflammation, which in turn aggravated bronchial edema.74 Blockade of VEGFR-3 also resulted in prolonged inflammation and edema in response to UVB irradiation.113 Thus, in contrast to VEGF-A, VEGF-C appears to promote lymphatic flow and contribute to the resolution of inflammation, suggesting that stimulation of lymphangiogenesis by VEGF-C may be beneficial for the treatment of certain inflammatory conditions.
Nevertheless, there might be some critical aspects to consider in this regard. In a mouse model of corneal inflammation, local blockade of VEGFR-3 signaling significantly suppressed trafficking of VEGFR-3-expressing dendritic cells from the cornea to the draining lymph nodes, which prevented the induction of delayed-type hypersensitivity (DTH) and the rejection of corneal transplants. This effect was dependent on dendritic cell trafficking per se rather than on reduced function of lymphatic vessels, since it occured earlier than a potential inhibition of lymphangiogenesis one might expect from anti-VEGFR-3 treatment.114
In the context of kidney transplant rejection, however, the lymphatic endothelium indeed appeared to actively foster the inflammatory process—through secretion of the chemokine CCL21 which attracts CCR7 positive immune cells, thereby facilitating their transport to the lymph node and the generation of adaptive immunity.104
Besides the well-characterized CCL21/CCR7 pathway and the mannose receptor, which was reported some years ago to be important for lymphocyte adhesion to lymphatic endothelium,115 novel mechanisms of immune cell entry into the afferent lymphatic vessels are currently being unraveled. Similar to blood vascular endothelium, LECs can be activated by inflammatory cytokines to expose key leukocyte adhesion receptors, such as intercellular adhesion molecule-1 (ICAM-1), vascular cell adhesion molecule-1 (VCAM-1), and E-selectin, promoting leukocyte transmigration across lymphatic endothelium.116 Conversely, LYVE-1, which was suspected to have a role in hyaluronan-mediated leukocyte adhesion to lymphatic endothelium, is internalized and degraded upon LEC stimulation by proinflammatory cytokines.117 Finally, sphingosine 1-phosphate (S1P) present in inflamed peripheral tissues was recently suggested to inhibit T-cell entry into afferent lymphatics through the S1P receptor 1 expressed on T-lymphocytes.118
The role of lymphatic vessels in cancer
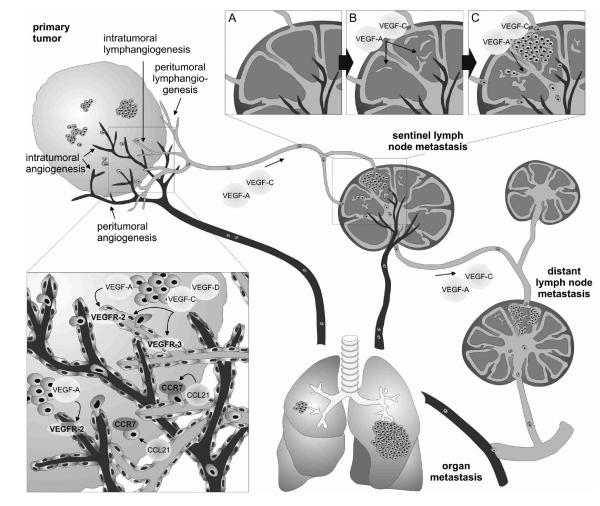
Lymphatic vessels as routes for trafficking through the body are exploited not only by immune cells but also by cancer cells. Metastasis to regional lymph nodes represents the first step of tumor dissemination in many cancers and is an important prognostic indicator for disease progression. A number of studies in animal tumor models have established the concept that tumors—rather than just accidentally invading pre-existing lymphatic vessels in their vicinity—can actively induce tumor-associated lymphangiogenesis by secreting appropriate growth factors such as VEGF-C,119–121 VEGF-D,122 or VEGF-A,70 which promotes their spread to the draining (sentinel) lymph nodes and beyond. Tumor-derived factors, which could partially also be contributed by tumor-infiltrating macrophages,61 have moreover been found to induce expansion of the lymphatic network in the sentinel lymph nodes, even before the arrival of the metastatic cancer cells, as if to create a favorable environment for the future metastasis70,119,123 (Fig. 2). Once there, metastatic tumor cells continue to induce lymphatic vessel growth within sentinel lymph nodes, which results in increased drainage of growth factors to and lymphatic expansion in distant lymph nodes (Fig. 2). VEGF-C-induced lymph node lymphanigogenesis promoted squamous cell carcinoma metastasis to distant lymph nodes and the lung,119 indicating that lymph node lymphangiogenesis contributes to tumor metastasis beyond the sentinel node. Importantly, while the correlation between VEGF-C or -D expression and tumor metastasis was repeatedly confirmed also in human cancers (reviewed in 124) and the extent of tumor-associated lymphangiogenesis was established as a potent predictor of lymphnode metastasis in human melanoma,125,126 a significant correlation between sentinel lymph node lymphangiogenesis and distant lymph node metastasis was recently found in human breast cancer patients.127 Taken together, these findings indicate that lymphangiogenesis at the site of the primary tumor as well as in the draining lymph node actively contributes to metastatic cancer spread and its inhibition might be of interest for preventing tumor metastasis.
FIG. 2.
Tumor and lymph node lymphangiogenesis promotes cancer metastasis. Tumor-derived VEGF-A and/or VEGF-C/-D induce lymphangiogenesis at the site of the primary tumor and within sentinel LNs, even before metastasis, possibly preparing the LN for the arrival of metastatic tumor cells. Once in the sentinel LN, these cells maintain their lymphangiogenic activity, likely promoting further cancer spread to distant lymph nodes and organs. The lymphatic endothelial-derived chemokine CCL21 attracts CCR7-expressing cancer cells towards the lymphatic vessels, facilitating their entry into the lymphatic system. CCR7, CC-chemokine receptor 7; LN, lymph node; VEGF, vascular endothelial growth factor.
To this aim, suppressing the effects of VEGF-C and -D by blocking VEGFR-3 signaling appears to be most promising, given their crucial importance for lymphatic vessel growth. Indeed, reduction of tumor lymphangiogenesis and lymph node metastasis has been achieved by means of neutralizing antibodies against VEGFR-3 or its ligands,122,128 soluble VEGFR-3-Ig fusion protein,129,130 or small interfering RNA (siRNA)-mediated VEGF-C gene silencing.131 Confidence that inhibition of the VEGF-C/-D/VEGFR-3 pathway could be a safe anti-lymphangiogenic strategy was provided by the recent finding that it does not affect normal lymphatic vessels in adult mice.65 Meanwhile, increasing evidence points towards a role for VEGFR-3 also in tumor angiogenesis. While in normal adult tissues VEGFR-3 expression is restricted mainly to the lymphatic endothelium, it becomes re-expressed on angiogenic tumor blood vessels.132–134 Moreover, it has recently been shown that interfering with VEGFR-3 function by means of a blocking antibody inhibited tumor angiogenesis and thereby growth of tumor xenografts in mice,135 which might represent an additional beneficial effect of anti-VEGFR-3 therapy against cancer.
Because VEGF-C and -D can activate VEGFR-2 and VEGF-A promotes tumor lymphangiogenesis and metastasis, also blockade of VEGFR-2 results in moderate suppression of lymphangiogenesis and lymphatic metastases—in addition to inhibition of angiogenesis and tumor growth—and anti-VEGFR-2 and anti-VEGFR-3 antibodies in combination more potently decrease lymph node and lung metastases than either antibody alone.128
Another potential target for the inhibition of cancer metastasis via lymphatic vessels might be the non-kinase receptor neuropilin-2. It was recently reported to be expressed on tumor-activated but not quiescent adult lymphatic vessels. An antibody blocking its interaction with VEGF-C inhibited tumor lymphangiogenesis and reduced functional tumor-associated lymphatic vessels and lymphatic metastasis, while leaving normal, established lymphatics in adult mice unaffected.136
In addition to tumor cells secreting factors which promote the growth of lymphatic vessels, LECs might secrete factors which attract tumor cells towards the lymphatic vessels, and hence might be worth targeting. Since its receptor CCR7 is expressed by certain cancer cell lines, one such candidate could be the lymphatic chemokine CCL21, which is known to play a crucial role in the migration of activated dendritic cells through lymphatic vessels to regional lymph nodes. Indeed, CCL21 was shown to induce chemotaxis of CCR7-expressing metastatic human melanoma cells in vitro and in vivo,137 and overexpression of CCR7 promoted murine B16 melanoma metastasis to draining lymph nodes.138
Acknowledgments
Research from the authors’ laboratory is supported by National Institutes of Health grant CA69184, Swiss National Fund grant 3100A0-108207, Austrian Science Foundation grant S9408-B11, Cancer League Zurich, Oncosuisse and Commission of the European Communities grant LSHC-CT-2005-518178 (M.D.).
Footnotes
Disclosures Ms. Cueni and Dr. Detmar have no conflicts of interest or financial ties to disclose.
References
- 1.Asellius G. De lactibus sive lacteis venis. Mediolani; Milan: 1627. [Google Scholar]
- 2.Baluk P, Fuxe J, Hashizume H, Romano T, Lashnits E, Butz S, Vestweber D, Corada M, Molendini C, Dejana E, Mc-Donald DM. Functionally specialized junctions between endothelial cells of lymphatic vessels. J Exp Med. 2007;204:2349–2362. doi: 10.1084/jem.20062596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sabin FR. On the origin of the lymphatic system from the veins and the development of the lymph hearts and thoracic duct in the pig. Am J Anat. 1902;1:367–389. [Google Scholar]
- 4.Huntington GS, McClure CFW. The anatomy and development of the jugular lymph sacs in the domestic cat (Felis domestica) Anat Rec. 1908;2:1–18. [Google Scholar]
- 5.Wigle JT, Harvey N, Detmar M, Lagutina I, Grosveld G, Gunn MD, Jackson DG, Oliver G. An essential role for Prox1 in the induction of the lymphatic endothelial cell phenotype. Embo J. 2002;21:1505–1513. doi: 10.1093/emboj/21.7.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wigle JT, Oliver G. Prox1 function is required for the development of the murine lymphatic system. Cell. 1999;98:769–778. doi: 10.1016/s0092-8674(00)81511-1. [DOI] [PubMed] [Google Scholar]
- 7.Oliver G, Sosa-Pineda B, Geisendorf S, Spana EP, Doe CQ, Gruss P. Prox1, a prospero-related homeobox gene expressed during mouse development. Mech Dev. 1993;44:3–16. doi: 10.1016/0925-4773(93)90012-m. [DOI] [PubMed] [Google Scholar]
- 8.Srinivasan RS, Dillard ME, Lagutin OV, Lin FJ, Tsai S, Tsai MJ, Samokhvalov IM, Oliver G. Lineage tracing demonstrates the venous origin of the mammalian lymphatic vasculature. Genes Dev. 2007;21:2422–2432. doi: 10.1101/gad.1588407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yaniv K, Isogai S, Castranova D, Dye L, Hitomi J, Weinstein BM. Live imaging of lymphatic development in the zebrafish. Nat Med. 2006;12:711–716. doi: 10.1038/nm1427. [DOI] [PubMed] [Google Scholar]
- 10.Ny A, Koch M, Schneider M, Neven E, Tong RT, Maity S, Fischer C, Plaisance S, Lambrechts D, Heligon C, Terclavers S, Ciesiolka M, Kalin R, Man WY, Senn I, Wyns S, Lupu F, Brandli A, Vleminckx K, Collen D, Dewerchin M, Conway EM, Moons L, Jain RK, Carmeliet P. A genetic Xenopus laevis tadpole model to study lymphangiogenesis. Nat Med. 2005;11:998–1004. doi: 10.1038/nm1285. [DOI] [PubMed] [Google Scholar]
- 11.Schneider M, Othman-Hassan K, Christ B, Wilting J. Lymphangioblasts in the avian wing bud. Dev Dyn. 1999;216:311–319. doi: 10.1002/(SICI)1097-0177(199912)216:4/5<311::AID-DVDY1>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 12.Wilting J, Aref Y, Huang R, Tomarev SI, Schweigerer L, Christ B, Valasek P, Papoutsi M. Dual origin of avian lymphatics. Dev Biol. 2006;292:165–173. doi: 10.1016/j.ydbio.2005.12.043. [DOI] [PubMed] [Google Scholar]
- 13.Kreuger J, Nilsson I, Kerjaschki D, Petrova T, Alitalo K, Claesson-Welsh L. Early lymph vessel development from embryonic stem cells. Arterioscler Thromb Vasc Biol. 2006;26:1073–1078. doi: 10.1161/01.ATV.0000217610.58032.b7. [DOI] [PubMed] [Google Scholar]
- 14.Liersch R, Nay F, Lu L, Detmar M. Induction of lymphatic endothelial cell differentiation in embryoid bodies. Blood. 2006;107:1214–1216. doi: 10.1182/blood-2005-08-3400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Buttler K, Kreysing A, von Kaisenberg CS, Schweigerer L, Gale N, Papoutsi M, Wilting J. Mesenchymal cells with leukocyte and lymphendothelial characteristics in murine embryos. Dev Dyn. 2006;235:1554–1562. doi: 10.1002/dvdy.20737. [DOI] [PubMed] [Google Scholar]
- 16.Karkkainen MJ, Haiko P, Sainio K, Partanen J, Taipale J, Petrova TV, Jeltsch M, Jackson DG, Talikka M, Rauvala H, Betsholtz C, Alitalo K. Vascular endothelial growth factor C is required for sprouting of the first lymphatic vessels from embryonic veins. Nat Immunol. 2004;5:74–80. doi: 10.1038/ni1013. [DOI] [PubMed] [Google Scholar]
- 17.Baldwin ME, Halford MM, Roufail S, Williams RA, Hibbs ML, Grail D, Kubo H, Stacker SA, Achen MG. Vascular endothelial growth factor D is dispensable for development of the lymphatic system. Mol Cell Biol. 2005;25:2441–2449. doi: 10.1128/MCB.25.6.2441-2449.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Avantaggiato V, Orlandini M, Acampora D, Oliviero S, Simeone A. Embryonic expression pattern of the murine figf gene, a growth factor belonging to platelet-derived growth factor/vascular endothelial growth factor family. Mech Dev. 1998;73:221–224. doi: 10.1016/s0925-4773(98)00049-5. [DOI] [PubMed] [Google Scholar]
- 19.Dumont DJ, Jussila L, Taipale J, Lymboussaki A, Mustonen T, Pajusola K, Breitman M, Alitalo K. Cardiovascular failure in mouse embryos deficient in VEGF receptor-3. Science. 1998;282:946–949. doi: 10.1126/science.282.5390.946. [DOI] [PubMed] [Google Scholar]
- 20.Kaipainen A, Korhonen J, Mustonen T, van Hinsbergh VW, Fang GH, Dumont D, Breitman M, Alitalo K. Expression of the fms-like tyrosine kinase 4 gene becomes restricted to lymphatic endothelium during development. Proc Natl Acad Sci USA. 1995;92:3566–3570. doi: 10.1073/pnas.92.8.3566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Banerji S, Ni J, Wang SX, Clasper S, Su J, Tammi R, Jones M, Jackson DG. LYVE-1, a new homologue of the CD44 glycoprotein, is a lymph-specific receptor for hyaluronan. J Cell Biol. 1999;144:789–801. doi: 10.1083/jcb.144.4.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gale NW, Prevo R, Espinosa J, Ferguson DJ, Dominguez MG, Yancopoulos GD, Thurston G, Jackson DG. Normal lymphatic development and function in mice deficient for the lymphatic hyaluronan receptor LYVE-1. Mol Cell Biol. 2007;27:595–604. doi: 10.1128/MCB.01503-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang SS, Liu IH, Smith T, Shah MR, Johnson FE, Huang JS. CRSBP-1/LYVE-l-null mice exhibit identifiable morphological and functional alterations of lymphatic capillary vessels. FEBS Lett. 2006;580:6259–6268. doi: 10.1016/j.febslet.2006.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abtahian F, Guerriero A, Sebzda E, Lu MM, Zhou R, Mocsai A, Myers EE, Huang B, Jackson DG, Ferrari VA, Tybulewicz V, Lowell CA, Lepore JJ, Koretzky GA, Kahn ML. Regulation of blood and lymphatic vascular separationby signaling proteins SLP-76 and Syk. Science. 2003;299:247–251. doi: 10.1126/science.1079477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sebzda E, Hibbard C, Sweeney S, Abtahian F, Bezman N, Clemens G, Maltzman JS, Cheng L, Liu F, Turner M, Tybulewicz V, Koretzky GA, Kahn ML. Syk and Slp-76 mutant mice reveal a cell-autonomous hematopoietic cell contribution to vascular development. Dev Cell. 2006;11:349–361. doi: 10.1016/j.devcel.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 26.Taniguchi K, Kohno R, Ayada T, Kato R, Ichiyama K, Morisada T, Oike Y, Yonemitsu Y, Maehara Y, Yoshimura A. Spreds are essential for embryonic lymphangiogenesis by regulating vascular endothelial growth factor receptor 3 signaling. Mol Cell Biol. 2007;27:4541–4550. doi: 10.1128/MCB.01600-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Backhed F, Crawford PA, O’Donnell D, Gordon JI. Postnatal lymphatic partitioning from the blood vasculature in the small intestine requires fasting-induced adipose factor. Proc Natl Acad Sci USA. 2007;104:606–611. doi: 10.1073/pnas.0605957104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Petrova TV, Karpanen T, Norrmen C, Mellor R, Tamakoshi T, Finegold D, Ferrell R, Kerjaschki D, Mortimer P, Yla-Herttuala S, Miura N, Alitalo K. Defective valves and abnormal mural cell recruitment underlie lymphatic vascular failure in lymphedema distichiasis. Nat Med. 2004;10:974–981. doi: 10.1038/nm1094. [DOI] [PubMed] [Google Scholar]
- 29.Gale NW, Thurston G, Hackett SF, Renard R, Wang Q, Mc-Clain J, Martin C, Witte C, Witte MH, Jackson D, Suri C, Campochiaro PA, Wiegand SJ, Yancopoulos GD. Angiopoietin-2 is required for postnatal angiogenesis and lymphatic patterning, and only the latter role is rescued by Angiopoietin-1. Dev Cell. 2002;3:411–423. doi: 10.1016/s1534-5807(02)00217-4. [DOI] [PubMed] [Google Scholar]
- 30.Shimoda H, Bernas MJ, Witte MH, Gale NW, Yancopoulos GD, Kato S. Abnormal recruitment of periendothelial cells to lymphatic capillaries in digestive organs of angiopoietin-2-deficient mice. Cell Tissue Res. 2007;328:329–337. doi: 10.1007/s00441-006-0360-8. [DOI] [PubMed] [Google Scholar]
- 31.Yuan L, Moyon D, Pardanaud L, Breant C, Karkkainen MJ, Alitalo K, Eichmann A. Abnormal lymphatic vessel development in neuropilin 2 mutant mice. Development. 2002;129:4797–4806. doi: 10.1242/dev.129.20.4797. [DOI] [PubMed] [Google Scholar]
- 32.Makinen T, Adams RH, Bailey J, Lu Q, Ziemiecki A, Alitalo K, Klein R, Wilkinson GA. PDZ interaction site in ephrinB2 is required for the remodeling of lymphatic vasculature. Genes Dev. 2005;19:397–410. doi: 10.1101/gad.330105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Breiteneder-Geleff S, Soleiman A, Kowalski H, Horvat R, Amann G, Kriehuber E, Diem K, Weninger W, Tschachler E, Alitalo K, Kerjaschki D. Angiosarcomas express mixed endothelial phenotypes of blood and lymphatic capillaries: podoplanin as a specific marker for lymphatic endothelium. Am J Pathol. 1999;154:385–394. doi: 10.1016/S0002-9440(10)65285-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hirakawa S, Hong YK, Harvey N, Schacht V, Matsuda K, Libermann T, Detmar M. Identification of vascular lineage-specific genes by transcriptional profiling of isolated blood vascular and lymphatic endothelial cells. Am J Pathol. 2003;162:575–586. doi: 10.1016/S0002-9440(10)63851-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kriehuber E, Breiteneder-Geleff S, Groeger M, Soleiman A, Schoppmann SF, Stingl G, Kerjaschki D, Maurer D. Isolation and characterization of dermal lymphatic and blood endothelial cells reveal stable and functionally specialized cell lineages. J Exp Med. 2001;194:797–808. doi: 10.1084/jem.194.6.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Petrova TV, Makinen T, Makela TP, Saarela J, Virtanen I, Ferrell RE, Finegold DN, Kerjaschki D, Yla-Herttuala S, Alitalo K. Lymphatic endothelial reprogramming of vascular endothelial cells by the Prox-1 homeobox transcription factor. Embo J. 2002;21:4593–4599. doi: 10.1093/emboj/cdf470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schacht V, Ramirez MI, Hong YK, Hirakawa S, Feng D, Harvey N, Williams M, Dvorak AM, Dvorak HF, Oliver G, Detmar M. T1alpha/podoplanin deficiency disrupts normal lymphatic vasculature formation and causes lymphedema. Embo J. 2003;22:3546–3556. doi: 10.1093/emboj/cdg342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ramirez MI, Millien G, Hinds A, Cao Y, Seldin DC, Williams MC. T1alpha, a lung type I cell differentiation gene, is required for normal lung cell proliferation and alveolus formation at birth. Dev Biol. 2003;256:61–72. doi: 10.1016/s0012-1606(02)00098-2. [DOI] [PubMed] [Google Scholar]
- 39.Kato Y, Fujita N, Kunita A, Sato S, Kaneko M, Osawa M, Tsuruo T. Molecular identification of Aggrus/T1alpha as a platelet aggregation-inducing factor expressed in colorectal tumors. J Biol Chem. 2003;278:51599–51605. doi: 10.1074/jbc.M309935200. [DOI] [PubMed] [Google Scholar]
- 40.Suzuki-Inoue K, Kato Y, Inoue O, Kaneko MK, Mishima K, Yatomi Y, Yamazaki Y, Narimatsu H, Ozaki Y. Involvement of the snake toxin receptor CLEC-2, in podoplanin-mediated platelet activation, by cancer cells. J Biol Chem. 2007;282:25993–26001. doi: 10.1074/jbc.M702327200. [DOI] [PubMed] [Google Scholar]
- 41.Martin-Villar E, Megias D, Castel S, Yurrita MM, Vilaro S, Quintanilla M. Podoplanin binds ERM proteins to activate RhoA and promote epithelial-mesenchymal transition. J Cell Sci. 2006;119:4541–4553. doi: 10.1242/jcs.03218. [DOI] [PubMed] [Google Scholar]
- 42.Wicki A, Lehembre F, Wick N, Hantusch B, Kerjaschki D, Christofori G. Tumor invasion in the absence of epithelial-mesenchymal transition: podoplanin-mediated remodeling of the actin cytoskeleton. Cancer Cell. 2006;9:261–272. doi: 10.1016/j.ccr.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 43.Suzuki-Inoue K, Fuller GL, Garcia A, Eble JA, Pohlmann S, Inoue O, Gartner TK, Hughan SC, Pearce AC, Laing GD, Theakston RD, Schweighoffer E, Zitzmann N, Morita T, Tybulewicz VL, Ozaki Y, Watson SP. A novel Syk-dependent mechanism of platelet activation by the C-type lectin receptor CLEC-2. Blood. 2006;107:542–549. doi: 10.1182/blood-2005-05-1994. [DOI] [PubMed] [Google Scholar]
- 44.Gunn MD, Tangemann K, Tam C, Cyster JG, Rosen SD, Williams LT. A chemokine expressed in lymphoid high endothelial venules promotes the adhesion and chemotaxis of naive T lymphocytes. Proc Natl Acad Sci USA. 1998;95:258–263. doi: 10.1073/pnas.95.1.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Karpanen T, Heckman CA, Keskitalo S, Jeltsch M, Ollila H, Neufeld G, Tamagnone L, Alitalo K. Functional interaction of VEGF-C and VEGF-D with neuropilin receptors. Faseb J. 2006;20:1462–1472. doi: 10.1096/fj.05-5646com. [DOI] [PubMed] [Google Scholar]
- 46.Sulpice E, Plouet J, Berge M, Allanic D, Tobelem G, Merkulova-Rainon T. Neuropilin-1 and neuropilin-2 act as coreceptors, potentiating proangiogenic activity. Blood. 2008;111:2036–2045. doi: 10.1182/blood-2007-04-084269. [DOI] [PubMed] [Google Scholar]
- 47.Makinen T, Veikkola T, Mustjoki S, Karpanen T, Catimel B, Nice EC, Wise L, Mercer A, Kowalski H, Kerjaschki D, Stacker SA, Achen MG, Alitalo K. Isolated lymphatic endothelial cells transduce growth, survival and migratory signals via the VEGF-C/D receptor VEGFR-3. Embo J. 2001;20:4762–4773. doi: 10.1093/emboj/20.17.4762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Roesli C, Mumprecht V, Neri D, Detmar M. Identification of the surface-accessible, lineage-specific vascular proteome by two-dimensional peptide mapping. Faseb J. 2008 doi: 10.1096/fj.07-100529. [DOI] [PubMed] [Google Scholar]
- 49.Amatschek S, Kriehuber E, Bauer W, Reininger B, Meraner P, Wolpl A, Schweifer N, Haslinger C, Stingl G, Maurer D. Blood and lymphatic endothelial cell-specific differentiation programs are stringently controlled by the tissue environment. Blood. 2007;109:4777–4785. doi: 10.1182/blood-2006-10-053280. [DOI] [PubMed] [Google Scholar]
- 50.Wick N, Saharinen P, Saharinen J, Gurnhofer E, Steiner CW, Raab I, Stokic D, Giovanoli P, Buchsbaum S, Burchard A, Thurner S, Alitalo K, Kerjaschki D. Transcriptomal comparison of human dermal lymphatic endothelial cells ex vivo and in vitro. Physiol Genomics. 2007;28:179–192. doi: 10.1152/physiolgenomics.00037.2006. [DOI] [PubMed] [Google Scholar]
- 51.Hong YK, Harvey N, Noh YH, Schacht V, Hirakawa S, Detmar M, Oliver G. Prox1 is a master control gene in the program specifying lymphatic endothelial cell fate. Dev Dyn. 2002;225:351–357. doi: 10.1002/dvdy.10163. [DOI] [PubMed] [Google Scholar]
- 52.Huang XZ, Wu JF, Ferrando R, Lee JH, Wang YL, Farese RV, Jr, Sheppard D. Fatal bilateral chylothorax in mice lacking the integrin alpha9beta1. Mol Cell Biol. 2000;20:5208–5215. doi: 10.1128/mcb.20.14.5208-5215.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vlahakis NE, Young BA, Atakilit A, Sheppard D. The lymphangiogenic vascular endothelial growth factors VEGF-C and -D are ligands for the integrin alpha9beta1. J Biol Chem. 2005;280:4544–4552. doi: 10.1074/jbc.M412816200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mishima K, Watabe T, Saito A, Yoshimatsu Y, Imaizumi N, Masui S, Hirashima M, Morisada T, Oike Y, Araie M, Niwa H, Kubo H, Suda T, Miyazono K. Prox1 induces lymphatic endothelial differentiation via integrin alpha9 and other signaling cascades. Mol Biol Cell. 2007;18:1421–1429. doi: 10.1091/mbc.E06-09-0780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dadras SS, Skrzypek A, Nguyen L, Shin J, Schulz MMP, Arbiser J, Mihm MC, Detmar M. Prox-1 promotes invasion of kaposiform hemangioendotheliomas. J Invest Dermatol. 2008 Jun 26; doi: 10.1038/jid.2008.176. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 56.Salven P, Mustjoki S, Alitalo R, Alitalo K, Rafii S. VEGFR-3 and CD133 identify a population of CD34+ lymphatic/vascular endothelial precursor cells. Blood. 2003;101:168–172. doi: 10.1182/blood-2002-03-0755. [DOI] [PubMed] [Google Scholar]
- 57.He Y, Rajantie I, Ilmonen M, Makinen T, Karkkainen MJ, Haiko P, Salven P, Alitalo K. Preexisting lymphatic endothelium but not endothelial progenitor cells are essential for tumor lymphangiogenesis and lymphatic metastasis. Cancer Res. 2004;64:3737–3740. doi: 10.1158/0008-5472.CAN-04-0088. [DOI] [PubMed] [Google Scholar]
- 58.Religa P, Cao R, Bjorndahl M, Zhou Z, Zhu Z, Cao Y. Presence of bone marrow-derived circulating progenitor endothelial cells in the newly formed lymphatic vessels. Blood. 2005;106:4184–4190. doi: 10.1182/blood-2005-01-0226. [DOI] [PubMed] [Google Scholar]
- 59.Maruyama K, Ii M, Cursiefen C, Jackson DG, Keino H, Tomita M, Van Rooijen N, Takenaka H, D’Amore PA, Stein-Streilein J, Losordo DW, Streilein JW. Inflammation-induced lymphangiogenesis in the cornea arises from CD11b-positive macrophages. J Clin Invest. 2005;115:2363–2372. doi: 10.1172/JCI23874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kerjaschki D, Huttary N, Raab I, Regele H, Bojarski-Nagy K, Bartel G, Krober SM, Greinix H, Rosenmaier A, Karlhofer F, Wick N, Mazal PR. Lymphatic endothelial progenitor cells contribute to de novo lymphangiogenesis in human renal transplants. Nat Med. 2006;12:230–234. doi: 10.1038/nm1340. [DOI] [PubMed] [Google Scholar]
- 61.Schoppmann SF, Birner P, Stockl J, Kalt R, Ullrich R, Caucig C, Kriehuber E, Nagy K, Alitalo K, Kerjaschki D. Tumor-associated macrophages express lymphatic endothelial growth factors and are related to peritumoral lymphangiogenesis. Am J Pathol. 2002;161:947–956. doi: 10.1016/S0002-9440(10)64255-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cursiefen C, Chen L, Borges LP, Jackson D, Cao J, Radziejewski C, D’Amore PA, Dana MR, Wiegand SJ, Streilein JW. VEGF-A stimulates lymphangiogenesis and hemangiogenesis in inflammatory neovascularization via macrophage recruitment. J Clin Invest. 2004;113:1040–1050. doi: 10.1172/JCI20465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jeltsch M, Kaipainen A, Joukov V, Meng X, Lakso M, Rauvala H, Swartz M, Fukumura D, Jain RK, Alitalo K. Hyperplasia of lymphatic vessels in VEGF-C transgenic mice. Science. 1997;276:1423–1425. doi: 10.1126/science.276.5317.1423. [DOI] [PubMed] [Google Scholar]
- 64.Veikkola T, Jussila L, Makinen T, Karpanen T, Jeltsch M, Petrova TV, Kubo H, Thurston G, McDonald DM, Achen MG, Stacker SA, Alitalo K. Signalling via vascular endothelial growth factor receptor-3 is sufficient for lymphangiogenesis in transgenic mice. Embo J. 2001;20:1223–1231. doi: 10.1093/emboj/20.6.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Karpanen T, Wirzenius M, Makinen T, Veikkola T, Haisma HJ, Achen MG, Stacker SA, Pytowski B, Yla-Herttuala S, Alitalo K. Lymphangiogenic growth factor responsiveness is modulated by postnatal lymphatic vessel maturation. Am J Pathol. 2006;169:708–718. doi: 10.2353/ajpath.2006.051200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Achen MG, Jeltsch M, Kukk E, Makinen T, Vitali A, Wilks AF, Alitalo K, Stacker SA. Vascular endothelial growth factor D (VEGF-D) is a ligand for the tyrosine kinases VEGF receptor 2 (Flk1) and VEGF receptor 3 (Flt4) Proc Natl Acad Sci USA. 1998;95:548–553. doi: 10.1073/pnas.95.2.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Joukov V, Pajusola K, Kaipainen A, Chilov D, Lahtinen I, Kukk E, Saksela O, Kalkkinen N, Alitalo K. A novel vascular endothelial growth factor, VEGF-C, is a ligand for the Flt4 (VEGFR-3) and KDR (VEGFR-2) receptor tyrosine kinases. Embo J. 1996;15:1751. [PMC free article] [PubMed] [Google Scholar]
- 68.Joukov V, Sorsa T, Kumar V, Jeltsch M, Claesson-Welsh L, Cao Y, Saksela O, Kalkkinen N, Alitalo K. Proteolytic processing regulates receptor specificity and activity of VEGF-C. Embo J. 1997;16:3898–3911. doi: 10.1093/emboj/16.13.3898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yamada Y, Nezu J, Shimane M, Hirata Y. Molecular cloning of a novel vascular endothelial growth factor, VEGF-D. Genomics. 1997;42:483–488. doi: 10.1006/geno.1997.4774. [DOI] [PubMed] [Google Scholar]
- 70.Hirakawa S, Kodama S, Kunstfeld R, Kajiya K, Brown LF, Detmar M. VEGF-A induces tumor and sentinel lymph node lymphangiogenesis and promotes lymphatic metastasis. J Exp Med. 2005;201:1089–1099. doi: 10.1084/jem.20041896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hong YK, Lange-Asschenfeldt B, Velasco P, Hirakawa S, Kunstfeld R, Brown LF, Bohlen P, Senger DR, Detmar M. VEGF-A promotes tissue repair-associated lymphatic vessel formation via VEGFR-2 and the alpha1beta1 and alpha2beta1 integrins. Faseb J. 2004;18:1111–1113. doi: 10.1096/fj.03-1179fje. [DOI] [PubMed] [Google Scholar]
- 72.Kunstfeld R, Hirakawa S, Hong YK, Schacht V, Lange-Asschenfeldt B, Velasco P, Lin C, Fiebiger E, Wei X, Wu Y, Hicklin D, Bohlen P, Detmar M. Induction of cutaneous delayed-type hypersensitivity reactions in VEGF-A transgenic mice results in chronic skin inflammation associated with persistent lymphatic hyperplasia. Blood. 2004;104:1048–1057. doi: 10.1182/blood-2003-08-2964. [DOI] [PubMed] [Google Scholar]
- 73.Nagy JA, Vasile E, Feng D, Sundberg C, Brown LF, Detmar MJ, Lawitts JA, Benjamin L, Tan X, Manseau EJ, Dvorak AM, Dvorak HF. Vascular permeability factor/vascular endothelial growth factor induces lymphangiogenesis as well as angiogenesis. J Exp Med. 2002;196:1497–14506. doi: 10.1084/jem.20021244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Baluk P, Tammela T, Ator E, Lyubynska N, Achen MG, Hicklin DJ, Jeltsch M, Petrova TV, Pytowski B, Stacker SA, Yla-Herttuala S, Jackson DG, Alitalo K, McDonald DM. Pathogenesis of persistent lymphatic vessel hyperplasia in chronic airway inflammation. J Clin Invest. 2005;115:247–257. doi: 10.1172/JCI22037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wirzenius M, Tammela T, Uutela M, He Y, Odorisio T, Zambruno G, Nagy JA, Dvorak HF, Yla-Herttuala S, Shibuya M, Alitalo K. Distinct vascular endothelial growth factor signals for lymphatic vessel enlargement and sprouting. J Exp Med. 2007;204:1431–1440. doi: 10.1084/jem.20062642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Goldman J, Rutkowski JM, Shields JD, Pasquier MC, Cui Y, Schmokel HG, Willey S, Hicklin DJ, Pytowski B, Swartz MA. Cooperative and redundant roles of VEGFR-2 and VEGFR-3 signaling in adult lymphangiogenesis. Faseb J. 2007;21:1003–1012. doi: 10.1096/fj.06-6656com. [DOI] [PubMed] [Google Scholar]
- 77.Pytowski B, Goldman J, Persaud K, Wu Y, Witte L, Hicklin DJ, Skobe M, Boardman KC, Swartz MA. Complete and specific inhibition of adult lymphatic regeneration by a novel VEGFR-3 neutralizing antibody. J Natl Cancer Inst. 2005;97:14–21. doi: 10.1093/jnci/dji003. [DOI] [PubMed] [Google Scholar]
- 78.Morisada T, Oike Y, Yamada Y, Urano T, Akao M, Kubota Y, Maekawa H, Kimura Y, Ohmura M, Miyamoto T, Nozawa S, Koh GY, Alitalo K, Suda T. Angiopoietin-1 promotes LYVE-1-positive lymphatic vessel formation. Blood. 2005;105:4649–4656. doi: 10.1182/blood-2004-08-3382. [DOI] [PubMed] [Google Scholar]
- 79.Tammela T, Saaristo A, Lohela M, Morisada T, Tornberg J, Norrmen C, Oike Y, Pajusola K, Thurston G, Suda T, Yla-Herttuala S, Alitalo K. Angiopoietin-1 promotes lymphatic sprouting and hyperplasia. Blood. 2005;105:4642–4648. doi: 10.1182/blood-2004-08-3327. [DOI] [PubMed] [Google Scholar]
- 80.Kajiya K, Hirakawa S, Ma B, Drinnenberg I, Detmar M. Hepatocyte growth factor promotes lymphatic vessel formation and function. Embo J. 2005;24:2885–2895. doi: 10.1038/sj.emboj.7600763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cao R, Bjorndahl MA, Gallego MI, Chen S, Religa P, Hansen AJ, Cao Y. Hepatocyte growth factor is a lymphangiogenic factor with an indirect mechanism of action. Blood. 2006;107:3531–3536. doi: 10.1182/blood-2005-06-2538. [DOI] [PubMed] [Google Scholar]
- 82.Chang LK, Garcia-Cardena G, Farnebo F, Fannon M, Chen EJ, Butterfield C, Moses MA, Mulligan RC, Folkman J, Kaipainen A. Dose-dependent response of FGF-2 for lymphangiogenesis. Proc Natl Acad Sci USA. 2004;101:11658–116563. doi: 10.1073/pnas.0404272101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kubo H, Cao R, Brakenhielm E, Makinen T, Cao Y, Alitalo K. Blockade of vascular endothelial growth factor receptor-3 signaling inhibits fibroblast growth factor-2-induced lymphangiogenesis in mouse cornea. Proc Natl Acad Sci USA. 2002;99:8868–8873. doi: 10.1073/pnas.062040199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Shin JW, Min M, Larrieu-Lahargue F, Canron X, Kunstfeld R, Nguyen L, Henderson JE, Bikfalvi A, Detmar M, Hong YK. Prox1 promotes lineage-specific expression of FGF receptor-3 in lymphatic endothelium: a role for FGF signaling in lymphangiogenesis. Mol Biol Cell. 2006;17:576–584. doi: 10.1091/mbc.E05-04-0368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bjorndahl M, Cao R, Nissen LJ, Clasper S, Johnson LA, Xue Y, Zhou Z, Jackson D, Hansen AJ, Cao Y. Insulin-like growth factors 1 and 2 induce lymphangiogenesis in vivo. Proc Natl Acad Sci USA. 2005;102:15593–15598. doi: 10.1073/pnas.0507865102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cao R, Bjorndahl MA, Religa P, Clasper S, Garvin S, Galter D, Meister B, Ikomi F, Tritsaris K, Dissing S, Ohhashi T, Jackson DG, Cao Y. PDGF-BB induces intratumoral lymphangiogenesis and promotes lymphatic metastasis. Cancer Cell. 2004;6:333–345. doi: 10.1016/j.ccr.2004.08.034. [DOI] [PubMed] [Google Scholar]
- 87.Cueni LN, Detmar M. New insights into the molecular control of the lymphatic vascular system and its role in disease. J Invest Dermatol. 2006;126:2167–2177. doi: 10.1038/sj.jid.5700464. [DOI] [PubMed] [Google Scholar]
- 88.Banziger-Tobler NE, Halin C, Kajiya K, Detmar M. Growth hormone promotes lymphangiogenesis. Am J Pathol. 2008;773:586–597. doi: 10.2353/ajpath.2008.080060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Fritz-Six KL, Dunworth WP, Li M, Caron KM. Adrenomedullin signaling is necessary for murine lymphatic vascular development. J Clin Invest. 2008;118:40–50. doi: 10.1172/JCI33302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Irrthum A, Karkkainen MJ, Devriendt K, Alitalo K, Vikkula M. Congenital hereditary lymphedema caused by a mutation that inactivates VEGFR3 tyrosine kinase. Am J Hum Genet. 2000;67:295–301. doi: 10.1086/303019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Karkkainen MJ, Ferrell RE, Lawrence EC, Kimak MA, Levinson KL, McTigue MA, Alitalo K, Finegold DN. Missense mutations interfere with VEGFR-3 signalling in primary lymphoedema. Nat Genet. 2000;25:153–159. doi: 10.1038/75997. [DOI] [PubMed] [Google Scholar]
- 92.Karkkainen MJ, Saaristo A, Jussila L, Karila KA, Lawrence EC, Pajusola K, Bueler H, Eichmann A, Kauppinen R, Kettunen MI, Yla-Herttuala S, Finegold DN, Ferrell RE, Alitalo K. A model for gene therapy of human hereditary lymphedema. Proc Natl Acad Sci USA. 2001;98:12677–12682. doi: 10.1073/pnas.221449198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Fang J, Dagenais SL, Erickson RP, Arlt MF, Glynn MW, Gorski JL, Seaver LH, Glover TW. Mutations in FOXC2 (MFH-1), a forkhead family transcription factor, are responsible for the hereditary lymphedema-distichiasis syndrome. Am J Hum Genet. 2000;67:1382–1388. doi: 10.1086/316915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Irrthum A, Devriendt K, Chitayat D, Matthijs G, Glade C, Steijlen PM, Fryns JP, Van Steensel MA, Vikkula M. Mutations in the transcription factor gene SOX18 underlie recessive and dominant forms of hypotrichosis-lymphedema-telangiectasia. Am J Hum Genet. 2003;72:1470–1478. doi: 10.1086/375614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Pennisi D, Gardner J, Chambers D, Hosking B, Peters J, Muscat G, Abbott C, Koopman P. Mutations in Sox18 underlie cardiovascular and hair follicle defects in ragged mice. Nat Genet. 2000;24:434–437. doi: 10.1038/74301. [DOI] [PubMed] [Google Scholar]
- 96.Melrose WD. Lymphatic filariasis: new insights into an old disease. Int J Parasitol. 2002;32:947–960. doi: 10.1016/s0020-7519(02)00062-0. [DOI] [PubMed] [Google Scholar]
- 97.Szuba A, Skobe M, Karkkainen MJ, Shin WS, Beynet DP, Rockson NB, Dakhil N, Spilman S, Goris ML, Strauss HW, Quertermous T, Alitalo K, Rockson SG. Therapeutic lymphangiogenesis with human recombinant VEGF-C. Faseb J. 2002;16:1985–1987. doi: 10.1096/fj.02-0401fje. [DOI] [PubMed] [Google Scholar]
- 98.Saaristo A, Veikkola T, Tammela T, Enholm B, Karkkainen MJ, Pajusola K, Bueler H, Yla-Herttuala S, Alitalo K. Lymphangiogenic gene therapy with minimal blood vascular side effects. J Exp Med. 2002;196:719–730. doi: 10.1084/jem.20020587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Tammela T, Saaristo A, Holopainen T, Lyytikka J, Kotronen A, Pitkonen M, Abo-Ramadan U, Yla-Herttuala S, Petrova TV, Alitalo K. Therapeutic differentiation and maturation of lymphatic vessels after lymph node dissection and transplantation. Nat Med. 2007;13:1458–1466. doi: 10.1038/nm1689. [DOI] [PubMed] [Google Scholar]
- 100.Nougues J, Reyne Y, Dulor JP. Differentiation of rabbit adipocyte precursors in primary culture. Int J Obes. 1988;12:321–333. [PubMed] [Google Scholar]
- 101.Harvey NL, Srinivasan RS, Dillard ME, Johnson NC, Witte MH, Boyd K, Sleeman M, Oliver G. Lymphatic vascular defects promoted by Prox1 haploinsufficiency cause adult-onset obesity. Nat Genet. 2005;37:1072–1081. doi: 10.1038/ng1642. [DOI] [PubMed] [Google Scholar]
- 102.Kaiserling E, Krober S, Geleff S. Lymphatic vessels in the colonic mucosa in ulcerative colitis. Lymphology. 2003;36:52–61. [PubMed] [Google Scholar]
- 103.Zhang Q, Lu Y, Proulx ST, Guo R, Yao Z, Schwarz EM, Boyce BF, Xing L. Increased lymphangiogenesis in joints of mice with inflammatory arthritis. Arthritis Res Ther. 2007;9:R118. doi: 10.1186/ar2326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kerjaschki D, Regele HM, Moosberger I, Nagy-Bojarski K, Watschinger B, Soleiman A, Birner P, Krieger S, Hovorka A, Silberhumer G, Laakkonen P, Petrova T, Langer B, Raab I. Lymphatic neoangiogenesis in human kidney transplants is associated with immunologically active lymphocytic infiltrates. J Am Soc Nephrol. 2004;15:603–612. doi: 10.1097/01.asn.0000113316.52371.2e. [DOI] [PubMed] [Google Scholar]
- 105.Detmar M, Brown LF, Claffey KP, Yeo KT, Kocher O, Jackman RW, Berse B, Dvorak HF. Overexpression of vascular permeability factor/vascular endothelial growth factor and its receptors in psoriasis. J Exp Med. 1994;180:1141–1146. doi: 10.1084/jem.180.3.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Halin C, Tobler NE, Vigl B, Brown LF, Detmar M. VEGF-A produced by chronically inflamed tissue induces lymphangiogenesis in draining lymph nodes. Blood. 2007;110:3158–3167. doi: 10.1182/blood-2007-01-066811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Angeli V, Ginhoux F, Llodra J, Quemeneur L, Frenette PS, Skobe M, Jessberger R, Merad M, Randolph GJ. B cell-driven lymphangiogenesis in inflamed lymph nodes enhances dendritic cell mobilization. Immunity. 2006;24:203–215. doi: 10.1016/j.immuni.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 108.Kajiya K, Hirakawa S, Detmar M. Vascular endothelial growth factor-A mediates ultraviolet B-induced impairment of lymphatic vessel function. Am J Pathol. 2006;169:1496–1503. doi: 10.2353/ajpath.2006.060197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ristimaki A, Narko K, Enholm B, Joukov V, Alitalo K. Proinflammatory cytokines regulate expression of the lymphatic endothelial mitogen vascular endothelial growth factor-C. J Biol Chem. 1998;273:8413–8418. doi: 10.1074/jbc.273.14.8413. [DOI] [PubMed] [Google Scholar]
- 110.Paavonen K, Mandelin J, Partanen T, Jussila L, Li TF, Ristimaki A, Alitalo K, Konttinen YT. Vascular endothelial growth factors C and D and their VEGFR-2 and 3 receptors in blood and lymphatic vessels in healthy and arthritic synovium. J Rheumatol. 2002;29:39–45. [PubMed] [Google Scholar]
- 111.Proulx ST, Kwok E, You Z, Beck CA, Shealy DJ, Ritchlin CT, Boyce BF, Xing L, Schwarz EM. MRI and quantification of draining lymph node function in inflammatory arthritis. Ann N Y Acad Sci. 2007;1117:106–123. doi: 10.1196/annals.1402.016. [DOI] [PubMed] [Google Scholar]
- 112.Polzer K, Baeten D, Soleiman A, Distler J, Gerlag DM, Tak PP, Schett G, Zwerina J. TNF blockade increases lymphangiogenesis in murine and human arthritic joints. Ann Rheum Dis. 2008 doi: 10.1136/ard.2007.083394. [DOI] [PubMed] [Google Scholar]
- 113.Kajiya K, Detmar M. An important role of lymphatic vessels in the control of UVB-induced edema formation and inflammation. J Invest Dermatol. 2006;126:919–921. doi: 10.1038/sj.jid.5700126. [DOI] [PubMed] [Google Scholar]
- 114.Chen L, Hamrah P, Cursiefen C, Zhang Q, Pytowski B, Streilein JW, Dana MR. Vascular endothelial growth factor receptor-3 mediates induction of corneal alloimmunity. Nat Med. 2004;10:813–815. doi: 10.1038/nm1078. [DOI] [PubMed] [Google Scholar]
- 115.Irjala H, Johansson EL, Grenman R, Alanen K, Salmi M, Jalkanen S. Mannose receptor is a novel ligand for L-selectin and mediates lymphocyte binding to lymphatic endothelium. J Exp Med. 2001;194:1033–1042. doi: 10.1084/jem.194.8.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Johnson LA, Clasper S, Holt AP, Lalor PF, Baban D, Jackson DG. An inflammation-induced mechanism for leukocyte transmigration across lymphatic vessel endothelium. J Exp Med. 2006;203:2763–2777. doi: 10.1084/jem.20051759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Johnson LA, Prevo R, Clasper S, Jackson DG. Inflammation-induced uptake and degradation of the lymphatic endothelial hyaluronan receptor LYVE-1. J Biol Chem. 2007;282:33671–33680. doi: 10.1074/jbc.M702889200. [DOI] [PubMed] [Google Scholar]
- 118.Ledgerwood LG, Lal G, Zhang N, Garin A, Esses SJ, Ginhoux F, Merad M, Peche H, Lira SA, Ding Y, Yang Y, He X, Schuchman EH, Allende ML, Ochando JC, Bromberg JS. The sphingosine 1-phosphate receptor 1 causes tissue retention by inhibiting the entry of peripheral tissue T lymphocytes into afferent lymphatics. Nat Immunol. 2008;9:42–53. doi: 10.1038/ni1534. [DOI] [PubMed] [Google Scholar]
- 119.Hirakawa S, Brown LF, Kodama S, Paavonen K, Alitalo K, Detmar M. VEGF-C-induced lymphangiogenesis in sentinel lymph nodes promotes tumor metastasis to distant sites. Blood. 2007;109:1010–1017. doi: 10.1182/blood-2006-05-021758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Mandriota SJ, Jussila L, Jeltsch M, Compagni A, Baetens D, Prevo R, Banerji S, Huarte J, Montesano R, Jackson DG, Orci L, Alitalo K, Christofori G, Pepper MS. Vascular endothelial growth factor-C-mediated lymphangiogenesis promotes tumour metastasis. Embo J. 2001;20:672–682. doi: 10.1093/emboj/20.4.672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Skobe M, Hawighorst T, Jackson DG, Prevo R, Janes L, Velasco P, Riccardi L, Alitalo K, Claffey K, Detmar M. Induction of tumor lymphangiogenesis by VEGF-C promotes breast cancer metastasis. Nat Med. 2001;7:192–198. doi: 10.1038/84643. [DOI] [PubMed] [Google Scholar]
- 122.Stacker SA, Caesar C, Baldwin ME, Thornton GE, Williams RA, Prevo R, Jackson DG, Nishikawa S, Kubo H, Achen MG. VEGF-D promotes the metastatic spread of tumor cells via the lymphatics. Nat Med. 2001;7:186–191. doi: 10.1038/84635. [DOI] [PubMed] [Google Scholar]
- 123.Harrell MI, Iritani BM, Ruddell A. Tumor-induced sentinel lymph node lymphangiogenesis and increased lymph flow precede melanoma metastasis. Am J Pathol. 2007;170:774–786. doi: 10.2353/ajpath.2007.060761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Rinderknecht M, Detmar M. Tumor lymphangiogenesis and melanoma metastasis. J Cell Physiol. 2008;216:347–354. doi: 10.1002/jcp.21494. [DOI] [PubMed] [Google Scholar]
- 125.Dadras SS, Lange-Asschenfeldt B, Velasco P, Nguyen L, Vora A, Muzikansky A, Jahnke K, Hauschild A, Hirakawa S, Mihm MC, Detmar M. Tumor lymphangiogenesis predicts melanoma metastasis to sentinel lymph nodes. Mod Pathol. 2005;18:1232–1242. doi: 10.1038/modpathol.3800410. [DOI] [PubMed] [Google Scholar]
- 126.Dadras SS, Paul T, Bertoncini J, Brown LF, Muzikansky A, Jackson DG, Ellwanger U, Garbe C, Mihm MC, Detmar M. Tumor lymphangiogenesis: a novel prognostic indicator for cutaneous melanoma metastasis and survival. Am J Pathol. 2003;162:1951–1960. doi: 10.1016/S0002-9440(10)64328-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Van den Eynden GG, Vandenberghe MK, van Dam PJ, Colpaert CG, van Dam P, Dirix LY, Vermeulen PB, Van Marck EA. Increased sentinel lymph node lymphangiogenesis is associated with nonsentinel axillary lymph node involvement in breast cancer patients with a positive sentinel node. Clin Cancer Res. 2007;13:5391–5397. doi: 10.1158/1078-0432.CCR-07-1230. [DOI] [PubMed] [Google Scholar]
- 128.Roberts N, Kloos B, Cassella M, Podgrabinska S, Persaud K, Wu Y, Pytowski B, Skobe M. Inhibition of VEGFR-3 activation with the antagonistic antibody more potently suppresses lymph node and distant metastases than inactivation of VEGFR-2. Cancer Res. 2006;66:2650–2657. doi: 10.1158/0008-5472.CAN-05-1843. [DOI] [PubMed] [Google Scholar]
- 129.Karpanen T, Egeblad M, Karkkainen MJ, Kubo H, Yla-Herttuala S, Jaattela M, Alitalo K. Vascular endothelial growth factor C promotes tumor lymphangiogenesis and intra-lymphatic tumor growth. Cancer Res. 2001;61:1786–1790. [PubMed] [Google Scholar]
- 130.He Y, Kozaki K, Karpanen T, Koshikawa K, Yla-Herttuala S, Takahashi T, Alitalo K. Suppression of tumor lymphangiogenesis and lymph node metastasis by blocking vascular endothelial growth factor receptor 3 signaling. J Natl Cancer Inst. 2002;94:819–825. doi: 10.1093/jnci/94.11.819. [DOI] [PubMed] [Google Scholar]
- 131.Chen Z, Varney ML, Backora MW, Cowan K, Solheim JC, Talmadge JE, Singh RK. Down-regulation of vascular endothelial cell growth factor-C expression using small interfering RNA vectors in mammary tumors inhibits tumor lymphangiogenesis and spontaneous metastasis and enhances survival. Cancer Res. 2005;65:9004–9011. doi: 10.1158/0008-5472.CAN-05-0885. [DOI] [PubMed] [Google Scholar]
- 132.Kubo H, Fujiwara T, Jussila L, Hashi H, Ogawa M, Shimizu K, Awane M, Sakai Y, Takabayashi A, Alitalo K, Yamaoka Y, Nishikawa SI. Involvement of vascular endothelial growth factor receptor-3 in maintenance of integrity of endothelial cell lining during tumor angiogenesis. Blood. 2000;96:546–553. [PubMed] [Google Scholar]
- 133.Partanen TA, Alitalo K, Miettinen M. Lack of lymphatic vascular specificity of vascular endothelial growth factor receptor 3 in 185 vascular tumors. Cancer. 1999;86:2406–2412. [PubMed] [Google Scholar]
- 134.Valtola R, Salven P, Heikkila P, Taipale J, Joensuu H, Rehn M, Pihlajaniemi T, Weich H, deWaal R, Alitalo K. VEGFR-3 and its ligand VEGF-C are associated with angiogenesis in breast cancer. Am J Pathol. 1999;154:1381–1390. doi: 10.1016/S0002-9440(10)65392-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Laakkonen P, Waltari M, Holopainen T, Takahashi T, Pytowski B, Steiner P, Hicklin D, Persaud K, Tonra JR, Witte L, Alitalo K. Vascular endothelial growth factor receptor 3 is involved in tumor angiogenesis and growth. Cancer Res. 2007;67:593–599. doi: 10.1158/0008-5472.CAN-06-3567. [DOI] [PubMed] [Google Scholar]
- 136.Caunt M, Mak J, Liang WC, Stawicki S, Pan Q, Tong RK, Kowalski J, Ho C, Reslan HB, Ross J, Berry L, Kasman I, Zlot C, Cheng Z, Le Couter J, Filvaroff EH, Plowman G, Peale F, French D, Carano R, Koch AW, Wu Y, Watts RJ, Tessier-Lavigne M, Bagri A. Blocking neuropilin-2 function inhibits tumor cell metastasis. Cancer Cell. 2008;13:331–342. doi: 10.1016/j.ccr.2008.01.029. [DOI] [PubMed] [Google Scholar]
- 137.Shields JD, Emmett MS, Dunn DB, Joory KD, Sage LM, Rigby H, Mortimer PS, Orlando A, Levick JR, Bates DO. Chemokine-mediated migration of melanoma cells towards lymphatics–a mechanism contributing to metastasis. Oncogene. 2007;26:2997–3005. doi: 10.1038/sj.onc.1210114. [DOI] [PubMed] [Google Scholar]
- 138.Wiley HE, Gonzalez EB, Maki W, Wu MT, Hwang ST. Expression of CC chemokine receptor-7 and regional lymph node metastasis of B16 murine melanoma. J Natl Cancer Inst. 2001;93:1638–1643. doi: 10.1093/jnci/93.21.1638. [DOI] [PubMed] [Google Scholar]
- 139.Kriederman BM, Myloyde TL, Witte MH, Dagenais SL, Witte CL, Rennels M, Bernas MJ, Lynch MT, Erickson RP, Caulder MS, Miura N, Jackson D, Brooks BP, Glover TW. FOXC2 haploinsufficient mice are a model for human autosomal dominant lymphedema-distichiasis syndrome. Hum Mol Genet. 2003;12:1179–1185. doi: 10.1093/hmg/ddg123. [DOI] [PubMed] [Google Scholar]
- 140.Seo S, Fujita H, Nakano A, Kang M, Duarte A, Kume T. The forkhead transcription factors, Foxc1 and Foxc2, are required for arterial specification and lymphatic sprouting during vascular development. Dev Biol. 2006;294:458–470. doi: 10.1016/j.ydbio.2006.03.035. [DOI] [PubMed] [Google Scholar]
- 141.Hirashima M, Sano K, Morisada T, Murakami K, Rossant J, Suda T. Lymphatic vessel assembly is impaired in Aspp1-deficient mouse embryos. Dev Biol. 2008 doi: 10.1016/j.ydbio.2008.01.023. [DOI] [PubMed] [Google Scholar]
- 142.Danussi C, Spessotto P, Petrucco A, Wassermann B, Sabatelli P, Montesi M, Doliana R, Bressan GM, Colombatti A. Emilin1 deficiency causes structural and functional defects of lymphatic vasculature. Mol Cell Biol. 2008 doi: 10.1128/MCB.02062-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Ayadi A, Zheng H, Sobieszczuk P, Buchwalter G, Moerman P, Alitalo K, Wasylyk B. Net-targeted mutant mice develop a vascular phenotype and up-regulate egr-1. Embo J. 2001;20:5139–5152. doi: 10.1093/emboj/20.18.5139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Gupta S, Ramjaun AR, Haiko P, Wang Y, Warne PH, Nicke B, Nye E, Stamp G, Alitalo K, Downward J. Binding of ras to phosphoinositide 3-kinase p110alpha is required for ras-driven tumorigenesis in mice. Cell. 2007;129:957–968. doi: 10.1016/j.cell.2007.03.051. [DOI] [PubMed] [Google Scholar]
- 145.Gittenberger-De Groot AC, Van Den Akker NM, Bartelings MM, Webb S, Van Vugt JM, Haak MC. Abnormal lymphatic development in trisomy 16 mouse embryos precedes nuchal edema. Dev Dyn. 2004;230:378–384. doi: 10.1002/dvdy.20054. [DOI] [PubMed] [Google Scholar]