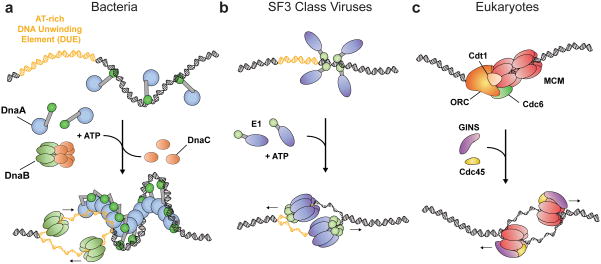
Figure 2.
Replication initiation mechanisms in cells and papilloma/polyomaviridae. (a) Bacteria. DnaA monomers are displayed as a blue oval (AAA+ domain) connected to a green oval (DNA binding domain). (Top)DnaA monomers associate with oriC. (Bottom) DnaA homo-oligomerizes in the presence of ATP, melting the DUE (yellow) and aiding the loading of the DnaB helicase (light green) with DnaC (orange). (b) SF3 viruses. Initiators are displayed as a purple oval (AAA+ domain) connected to a light green oval (DNA binding domain). (Top) monomers associate with the origin. (Bottom) Viral initiators melt the origin in the presence of ATP (yellow – DUE), eventually assembling into two hexameric helicases. (c) Eukaryotes. (Top) Activation of ORC by ATP stimulates formation of the pre-replicative complex (pre-RC), composed of ORC (orange), an MCM2-7 double-hexamer (red ovals), and the loader/chaperone proteins Cdc6 (green) and Cdt1 (tan). (Bottom) After loading, Cdc45 (yellow) and the GINS (purple) complex stimulate duplex melting by MCM2-7, giving rise to two hexameric helicases.