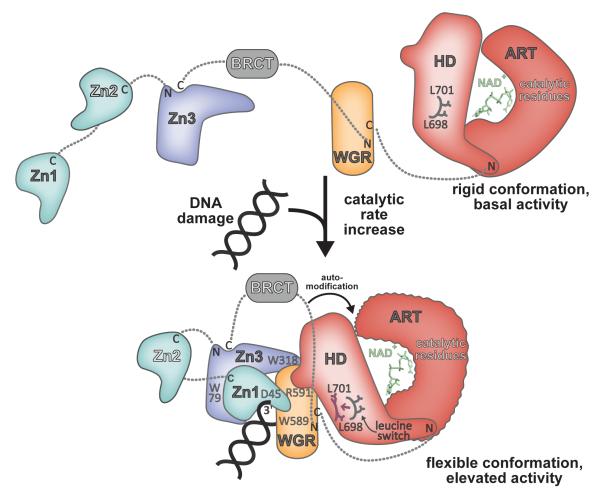
Figure 4. Model for DNA damage-dependent activation of PARP-1.
In the absence of DNA damage, PARP-1 domains exist in an extended, “beads-on-a-string” conformation. The HD serves as a modulator of PARP-1 activity, holding the ART in a rigid conformation. Upon detecting DNA damage the Zn1, Zn3, and WGR domains collapse together, forming a network of interdomain contacts that perturb the structure of the HD, displacing a “leucine switch” that decreases the stability of the catalytic domain and increases the catalytic activity. A more flexible, dynamic ART conformation is more efficient to perform the multi-step synthesis of poly(ADP-ribose). The collapsed conformation positions the automodication region adjacent to the catalytic domain, providing substrate specificity and contributing to an enhanced rate of poly(ADP-ribose) production.