Abstract
The most common form in bile duct cancers is a highly desmoplastic cancer with a growth pattern characterized by periductal extension and infiltration. The prognosis of bile duct cancers, especially hilar cholangiocarcinoma, is limited by tumor spread along the biliary tree leading to refractory obstructive cholestasis, cholangitis, and liver failure. Although biliary endoprosthesis improves occlusion rates and reduces the number of therapeutic interventions, median survival time is not ameliorated. Photodynamic therapy (PDT) is a local photochemical tumor treatment that consists of a photosensitizing agent in combination with laser irradiation of a distinct wavelength. Tumor ablation with PDT combined with biliary stenting reduces cholestasis and significantly improves median survival time in selected patients with bile duct cancers.
Keywords: Cholangiocarcinoma, Bile duct neoplasms, Endoprosthesis, Photodynamic therapy, Photosensitizing agents
INTRODUCTION
Bile duct cancer (BDC) is an uncommon malignant tumor that may arise anywhere that biliary epithelium is present from the ampulla of Vater to the smallest intrahepatic biliary radicles. BDCs are classified into three main categories: perihilar BDCs (also known as Klatskin's tumors), distal BDCs, and intrahepatic BDCs.1 Perihilar BDCs are further subclassified depending on the degree of the proximal tumor extension within the intrahepatic biliary radicles according to the classification proposed and later modified by Bismuth.2 Klatskin's tumors are the most common form of BDCs and account for approximately 70% of cases of BDCs. Distal and intrahepatic BDCs account for approximately 25% and 5% of cases, respectively.1
Complete resection with negative margins is the only treatment with the potential for cure, with 5-year survival rates of 20% to 40%.3,4 However, patients with cholangiocarcinoma (CC) usually present at an advanced stage, with more than 50% being unresectable at the time of diagnosis. Recently, two randomized controlled trials have shown a significant survival benefit in patients with unresectable CC treated with photodynamic therapy (PDT).5,6 One of these studies also showed a significant improvement in quality of life after PDT and stenting.6
PDT is based on the relatively specific accumulation of photosensitizers, such as porphyrins, in dysplastic or malignant cells. CC cell lines have shown favorable cellular uptake kinetics for sodium porfimer and excellent phototoxic cell damage in response to PDT in vitro and in vivo in a human CC xenograft model in nude mice.7,8 In this paper, we review recent advances of PDT in BDCs.
SPECIFIC FEATURES OF TUMOR BIOLOGY OF BDCS ARE SUITABLE FOR PDT
CC is characterized by a usually slow growth rate and a low propensity for metastasis. The most common form in BDCs is a highly desmoplastic cancer with a growth pattern characterized by periductal extension and infiltration (Fig. 1).9 This form of CC often obstructs bile ducts and encases blood vessels strangulating these structures mechanically and disrupting bile and blood flow, respectively. Because these cancers involve both intrahepatic and extrahepatic bile ducts, they will simply be referred to as ductal CCs: however, given their predilection to occur at the bifurcation of the right and left hepatic duct, others have referred to these cancers as perihilar CCs (Klatskin's tumor). In contrast, the other principal form of this disease grows as a mass lesion within the liver. This form of the disease will be referred to as intrahepatic CCs because they, in part, mimic hepatocelluar carcinomas. In addition to different growth pattern, current information suggests these two forms of CC may differ in their etiopathogenesis, risk factors, and perhaps molecular and genetic signatures.10
Fig. 1.
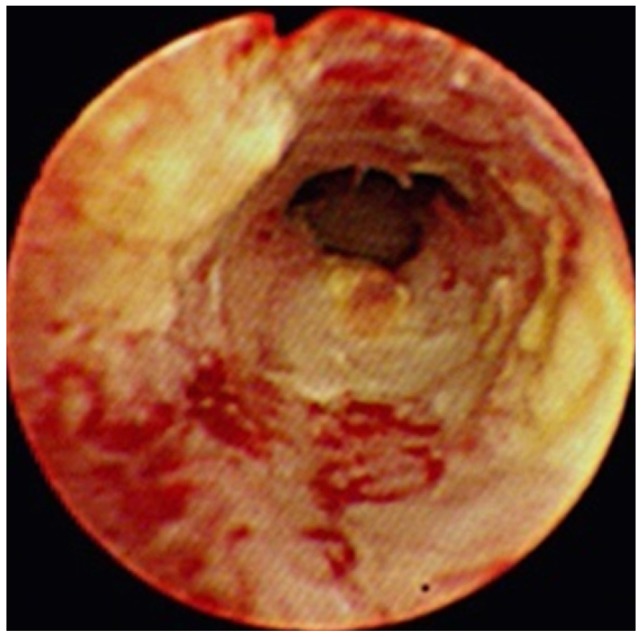
A sclerosing type of bile duct cancer. A cholangioscopy shows the luminal narrowing with a whitish mucosal discoloration and neovascularization.
Although nearly all CCs are well-differentiated adenocarcinoma, their tumor biology is a little different from other gastrointestinal adenocarcinoma, especially in hilar bile duct cancer (HBDC). In general, hematogenous spread of HBDC is rare, whereas nodal metastases may be present in up to one-third of cases.11 Extensive subepithelial tumors spread beyond the gross tumor margin is common, and longitudinal tumors may extend 15 to 20 mm proximally and 5 to 10 mm distally, depending on tumor type.12,13 The papillary variant composes 10% of all CCs and grows primary as an intraluminal soft, polypoid tumor with a limited propensity for transmural growth (Fig. 2). Tumor multicentricity occurs more frequently with the papillary variant and may be reflective of a field change in the biliary epithelium.11,14,15 The nodular variant occurs most commonly in the upper and mid bile duct and generally presents as a fibrotic mass with intraductal projections (Fig. 3). The sclerosing variant comprises 70% of all tumors at the hilum and histologically appears as annular thickening of the bile duct wall with both longitudinal and radial tumor infiltrations.
Fig. 2.
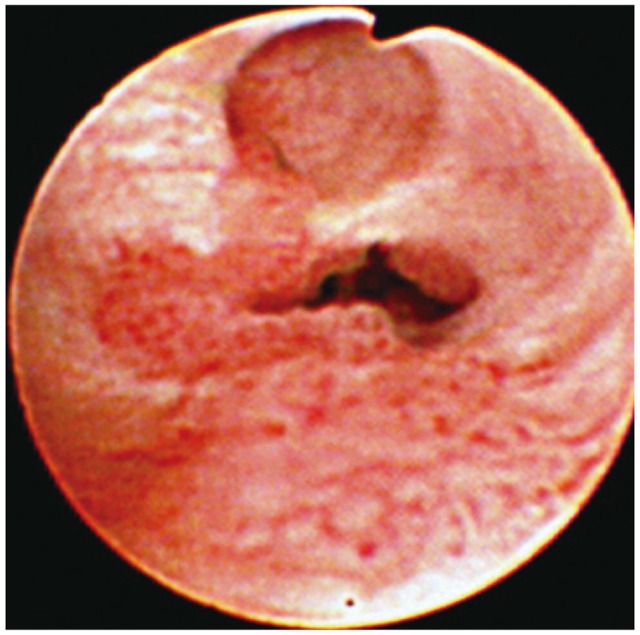
A papillary type of bile duct cancer. A cholangioscopy shows that slight papillary and mucosal nodularity is seen in the intrahepatic bile duct. In this type of tumor, the mucosal lesion may be minute and detectable only by careful cholangioscopic examination of the entire biliary tree.
Fig. 3.
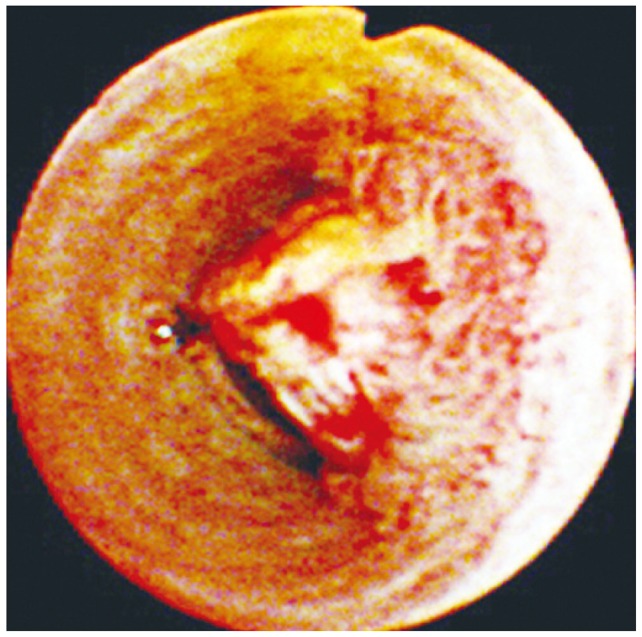
A nodular or polypoid type of bile duct cancer. A polypoid mass partially obstructs the lumen of the common hepatic duct. Mucosal irregularity and intense serpiginous neovascularization are clearly visible on the surface.
WHAT ARE THE APPROPRIATE INDICATIONS OF PDT IN BDCS?
Neoadjuvant PDT for hilar CC showed efficacious tumor destruction confined to the superficial 4- to 4.5-mm depth of the bile duct tumor and high tumor selectivity in the resected bile duct specimens.16 This cannot eradicate the primary tumor when tumor invasion extends to a depth of 7 to 9 mm. Therefore the appropriate indications of PDT in BDCs may be as follows: 1) sclerosing variant without hematogenous metastasis regardless of nodal metastases; 2) superficial spreading type with the papillary variant; and 3) R0 and R1 residual tumor after resection. The mass-forming intrahepatic CCs, intraductal mass form of BDCs and cases of hematogenous metastases are not indicated for PDT.
RATIONAL OF LOCAL TUMOR ABLATION IN BDCS
About 80% of BDCs, especially perihilar CCs, are adenocarcinomas that exhibit predominantly a longitudinal growth pattern along the biliary tree, most highly desmoplastic tumors with infiltration of adjacent nerve plexus and lymphatics.9
Even with an aggressive surgical approach only 33% to 50% are resectable, and in only 28% are negative histological margins obtained.17
The majority of patients with tumor stenoses in the distal and middle part of the bile duct cholestasis can be relieved quickly by stenting. The role of palliative intervention is limited in proximal BDCs. Independent of the type of stricture, technically successful endoprosthesis placement is possible in 84% to 96% of these patients.18,19 A successful drainage (bilirubin decrease >30% to 50%) is only achieved, however, in 69% to 91% of Bismuth type I and II stenosis and in 15% to 73% of Bismuth type III and IV tumors.18,20 Although metal stent insertion improves occlusion rates and reduces the number of therapeutic interventions, median survival time is not ameliorated.21,22 Attempts to affect tumor growth are made with radiotherapy or chemotherapy. Until now, however, chemotherapy is unsatisfactory in terms of survival times although chemotherapy with gemcitabine and platinum achieved a modest improvement in survival compared to other regimens.23 Whether radiotherapy is able to improve survival is still on debate. The retrospective comparative study of palliative radiation therapy showed no significantly improved median survival time (300 days vs. 210 days) between endoscopic biliary stenting with or without external-beam radiotherapy and internal 192Ir brachytherapy.24 Recently, stereotatic body radiotherapy may be a new promising option but has to be evaluated further.25
A treatment modality for local ablation of the primary tumor could improve the outcome of curative as well as palliative therapies. Palliative brachytherapy with only 192-iridium (dose of 35 Gy in 1-cm distance) did not prolong median survival time (4.3 to 5 months),26 but when combined with external beam radiotherapy (30 Gy), resulted in median survival times of 10 to 10.5 months.21 Another modality for local tumor ablation of CC is PDT.
PHOTODYNAMIC EFFECT IN BDCS
The tumor-selective enrichment of porfimer has been confirmed in human BDC biopsies analyzed with quantitative fluorescence microscopy. The average ratio of specific fluorescence in tumor versus normal tissue was 1.7 and 2.3 at 24 and 48 hours, respectively, after intravenous administration of porfimer.26 Porfimer enrichment in CC tissue should be adequate for PDT from day 1 to 4 after intravenous administration.
Wong Kee Song et al.7 showed a reduction of up to 60% of tumor volume after PDT with hematoporphyrin and with chlorine in nude mice inoculated with a human CC cell line. The PDT effect was evaluated in Buffalo female rats inoculated with rat hepatoma cells. The mean complete necrosis of a single session of PDT was 10.2 mm3.27 Neoadjuvant PDT of CC revealed that the tumoricidal depth of the PDT modality with porfimer was limited to 4- to 4.5-mm tissue penetration.28 Newer photosensitizers lead to deeper tumor necrosis and a shorter period of photosensitivity. Meso-tetrahydroxyphenyl chlorine is absorbed in the near-infrared spectrum (652 nm) and has the above characteristics.29
OUTCOME OF PDT FOR ADVANCED BDCS
In numerous controlled and uncontrolled studies, the combination of PDT and biliary drainage has shown promising results in patients with unresectable hilar CC (Table 1).
Table 1.
Outcome of PDT in the Studies
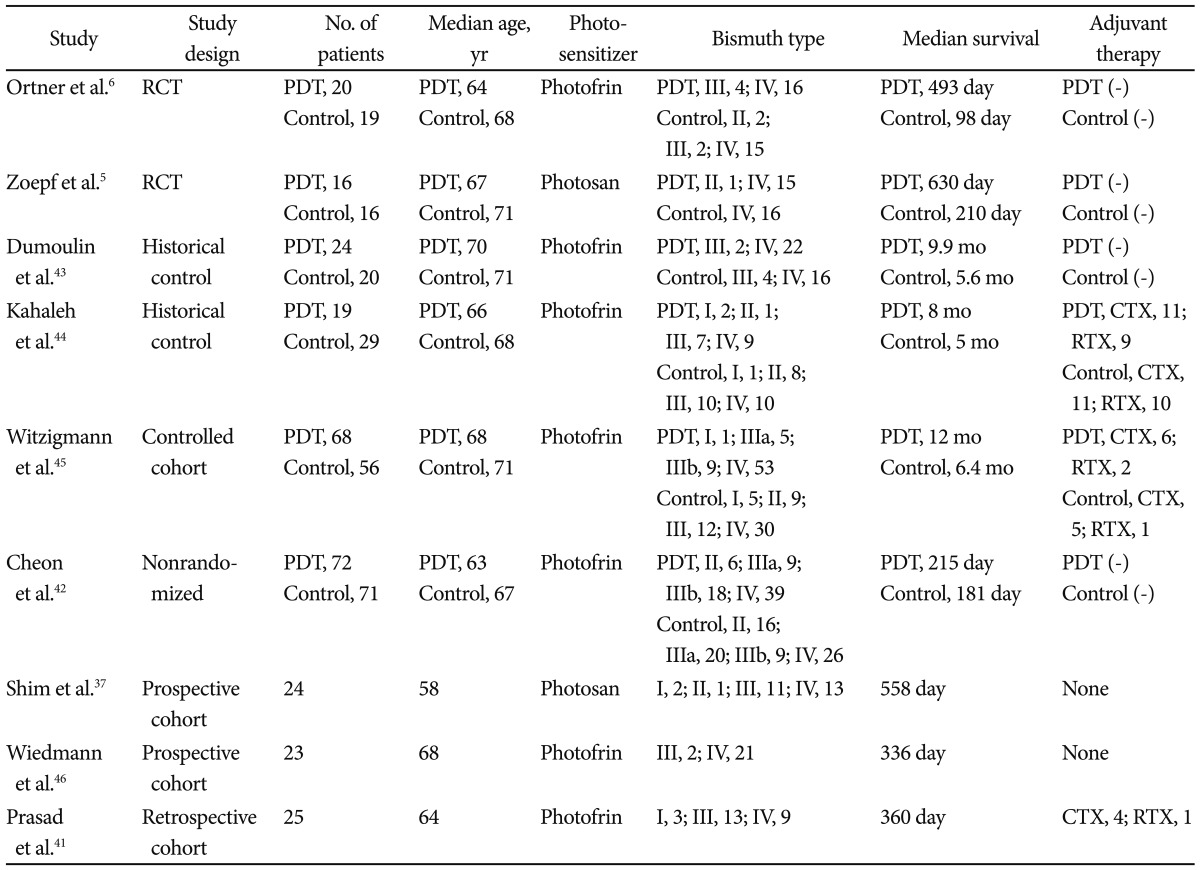
RCT, randomized controlled trial; PDT, photodynamic therapy; CTX, chemotherapy; RTX, radiation therapy.
The first case report of PDT for CC showed significant survival advantage over 4 years.30 In an early pilot study, Ortner et al.31 performed PDT in patients with unresectable CC Bismuth type III and IV who failed endoprostheses placement and had the poorest prognosis. Two days after intravenous application of a hematoporphyrin derivative Photofrin II, intraluminal photoactivation was performed cholangioscopically. Red light at 630 nm was emitted by an argon-dye laser and laser fibers of 400 µm core diameter with flexible cylindrical diffuser tips of 2.5 and 4 lengths were used. With output of 800 mW, the light flux was 310 and 190 mW/cm2, and by changing irradiation time the resulting dose was 180 J/cm2. Bilirubin serum levels declined from 318±72 to 103±35 µmol/L (p=0.0039) with no significant increase during the 2 monthly follow-ups. Quality of life indices improved dramatically (Karnofsky index 32.3%±8.13% to 68.9%±6.1%, p=0.0078; World Health Organization index 3.2±0.36 to 1.7±0.4, p=0.016; performance rating scale 13.6±1.6 to 5±0.93, p=0.0078) and remained stable during the follow-up. Thirty-day mortality was 0%, 1-year survival was 77.7%, and median survival time was 439 days.
In the prospective, open-label, randomized, multicenter study with a group sequential design comparing PDT in addition to stenting (group A) with stenting alone (group B) in patients with nonresectable CC was reported by Ortner et al.6 PDT resulted in prolongation of survival (group A, n=20, median 493 days; group B, n=19, median 98 days; p<0.0001). It also improved biliary drainage and quality of life. Authors concluded that PDT, given in addition to best supportive care, improves survival in patients with unresectable CC. The study was terminated prematurely because PDT was proved to be so superior to simple stenting treatment that further randomization was deemed unethical. Zoepf et al.5 conducted another prospective, randomized trial of 32 patients. The Photosan (SeeLab, Wesselburenerkoog, Germany) as a photosensitizer was administered. Median survival in PDT group was 630 days compared with 210 days for drainage alone. Compared with the study by Ortner et al.,6 the proportion of patients who received bilateral transpapillary stenting was low (10/18, 56%) and baseline patients' performance status was higher in Zoepf's study.32 Similar results were obtained by our previous study.7 Twenty patients who were treated with endoscopic biliary drainage alone (group A) and 27 patients treated with PDT under percutaneous cholangioscopy and additional percutaneous biliary drainage (group B) were analyzed retrospectively. The mean bilirubin level declined effectively in both group after treatment. One-year survival was 28% in group A, 52% in group B (p<0.05). Median survival time was 288 days in group A, 558 days in group B (p=0.0143).
One prospective study compared stenting with chemotherapy versus stenting with PDT for patients with hilar CC.33 The mean survival for the 17 patients treated with stenting and chemotherapy was 173 days, compared with 512 days for the 23 patients treated with stenting and PDT. As expected, 10 patients who were considered suitable for curative resection achieved longest survival of 1,278 days.
Recently, the effect of PDT on metal stent patency were analyzed in a retropsective study of 33 patients with unresectable CC by our group.34 We observed that the one session of PDT with metal stenting was associated with a significantly longer stent patency period (median 244±66 and 177±45 days, respectively, p=0.002) and patient survival (median 356±213 and 230±73 days, respectively, p=0.006) compared with the metal stent only group. The ability of PDT to destroy cancer cells and lessen cholestasis may prolong stent patency.
THE ROLE OF PDT FOR LOCAL RECURRENT TUMOR AFTER RESECTION OR AS NEOADJUVANT TREATMENT
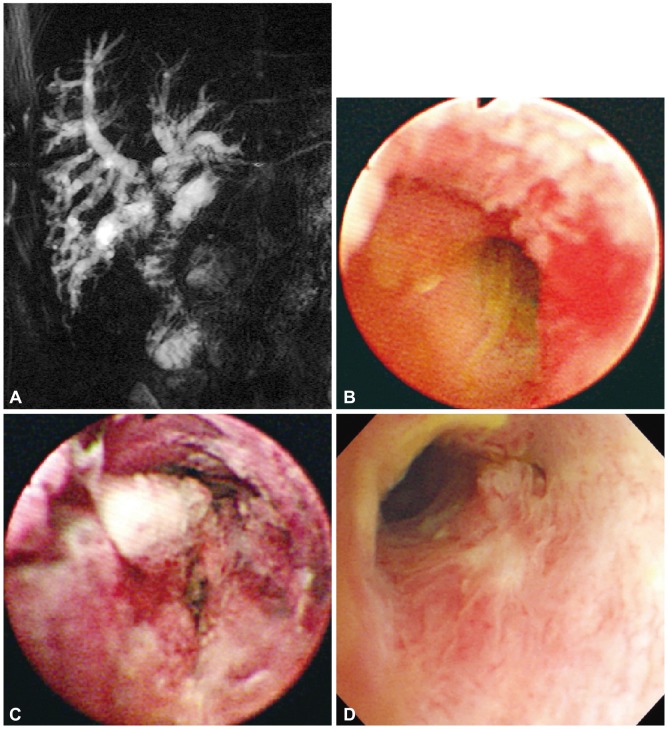
We experienced a case whose survival time prolonged remarkably after the application of PDT for a postoperative recurrent tumor (Fig. 4). A small (n=8) uncontrolled study showed marked destruction of the recurrent tumor; 75% of patients were disease free after 2 years.35
Fig. 4.
Photodynamic therapy (PDT) for postoperative recurrent tumor. A 76-year-old man was referred to our hospital with jaundice. He received Whipple's operation due to hilar cholangiocarcinoma 1 year ago. (A) Magnetic resonance imaging shows a contrast enhanced hilar mass with obstructing anastomosis site. (B) Cholangioscopy shows mucosal nodularity and neovascularization in the anastomosis site. Biopsy specimens revealed adenocarcinoma. (C) Two days after PDT. Cholangiocopy shows circumferential coagulation necrosis at the PDT-treated lesion. (D) One year after the PDT, recanalized anastomotic site and small papillary changes with no abnormal vessels can be seen on cholangioscopy. Cholangioscopic biopsy specimens revealed chronic nonspecific inflammation.
Neoadjuvant PDT was evaluated in seven patients with advanced Bismuth type III and IV carcinoma, which were thought to be unresectable.28 After PDT, a curative resection could be performed in all patients; 83% were recurrence free after 1 year and 5-year survival was 71%. No relevant side effects of PDT occurred except for a minor intraoperative phototoxicity in one patient.
ASSESSMENT OF RESPONSE TO PDT
Evaluation of the therapeutic effects of PDT for patients with CC presents a number of challenges. Ortner et al.6 evaluated the therapeutic effect of PDT in cases of advanced BDC by comparing the tumor length before and after PDT using the 'mother-baby' cholangioscopic technique; however, it has been argued that their assessment was insufficiently objective. Although the authors23 reported reduced serum bilirubin levels after PDT, plastic biliary endoprostheses were inserted in all patients following the PDT procedure, making it difficult to assess the direct effect of PDT in reducing serum bilirubin. However, the PDT group might have mainly benefited from the number of ERCP sessions. For this reason, we do not consider the serum bilirubin level to be an objective parameter for assessing the response to PDT. Ductal CCs characteristically spread along or within the intrahepatic bile ducts, making it difficult to define the response to PDT based on changes in the tumor mass by computed tomography (CT). Similarly, it may be difficult to obtain objective results when the evaluation of the response to PDT is based solely on the extent of reopening of an occluded segment of the bile duct, as has been done in some studies.36 Previously, we assessed the thickness of the tumor mass before and after PDT treatment, measuring the thickest part of the tumor before the treatment and every month thereafter.37 The mean thickness of the bile duct masses, as measured by intraductal ultrasound, decreased from 8.7±3.7 mm before PDT to 7.1±2.0 mm at 1 month (p=0.176), to 7.1±2.4 mm at 2 months (p=0.157), and declined significantly to 5.8±2.0 mm at 3 months (p=0.046) after PDT.
In murine cancer models, some investigators have found that serum interleukin (IL)-6 levels correlate with tumor burden.38,39 Goydos et al.40 reported that serum IL-6 levels were correlated with the tumor burden as measured by CT in patients with CC; 2 weeks after tumor resection in three of 15 patients, IL-6 levels had dropped to undetectable levels in two patients and by almost tenfold in the third. Our results similarly demonstrated that IL-6 was significantly reduced (38.2±9.9 pg/mL; p=0.008) 1 month after PDT compared with the pretreatment level (282.1±121.8 pg/mL); tumor thickness also decreased following PDT. In contrast, IL-6 levels had not changed 1 month after ERBD.39
FACTORS ASSOCIATED WITH INCREASED SURVIVAL AFTER PDT
Recently, factors associated with patients' survival after PDT were analyzed in two studies.41,42 Prasad et al.41 conducted a retrospective study of 25 patients with unresectable CC treated with PDT in addition to biliary decompression. The presence of a visible mass on imaging studies, a lower serum albumin level and longer time between diagnosis and PDT predicted a poorer survival rate. Similar results were obtained in our retrospective analysis of 143 patients with hilar CC.42 We treated 72 patients with PDT and 71 patients with endoprotheses alone. We found that patients treated with PDT lived longer than those who were not treated with PDT (9.8 months vs. 7.3 months). Furthermore, lower pre-PDT bilirubin level, multiple PDT treatments and shortened time to treatment after diagnosis were significant predictors of improved survival. These two studies indicate the importance of early PDT after the diagnosis of unresectable CC.
CONCLUSIONS
Patients with unresectable CC had so far a very short life expectancy. PDT is the first palliative treatment option that has shown its efficacy in two randomized prospective studies. PDT improves survival, jaundice, and quality of life, is well tolerated and can be repeated without losing its efficacy.43-46 PDT seems to be a promising therapeutic approach for unresectable CC. It combines the aim to treat cholestasis and to reduce tumor growth. PDT, therefore, should be considered as a standard care for the palliation of CC. If the results are confirmed one could think of trying new photosensitizers with greater penetration depth and shorter photosensitivity, or using better drug targeting or combination therapies to induce more tumor necrosis. As PDT treatment is not available in all centers, patients should be referred to a specialized center with PDT availability. It is still not known whether radiotherapy and/or chemotherapy further improve the fate of PDT patients. It is now necessary to strengthen these data in an extended randomized multicenter study. PDT for recurrent tumors after surgery and neoadjuvant PDT is still experimental.
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Nakeeb A, Pitt HA, Sohn TA, et al. Cholangiocarcinoma. A spectrum of intrahepatic, perihilar, and distal tumors. Ann Surg. 1996;224:463–473. doi: 10.1097/00000658-199610000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bismuth H, Castaing D, Traynor O. Resection or palliation: priority of surgery in the treatment of hilar cancer. World J Surg. 1988;12:39–47. doi: 10.1007/BF01658484. [DOI] [PubMed] [Google Scholar]
- 3.Rea DJ, Munoz-Juarez M, Farnell MB, et al. Major hepatic resection for hilar cholangiocarcinoma: analysis of 46 patients. Arch Surg. 2004;139:514–523. doi: 10.1001/archsurg.139.5.514. [DOI] [PubMed] [Google Scholar]
- 4.Jarnagin WR, Fong Y, DeMatteo RP, et al. Staging, resectability, and outcome in 225 patients with hilar cholangiocarcinoma. Ann Surg. 2001;234:507–517. doi: 10.1097/00000658-200110000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zoepf T, Jakobs R, Arnold JC, Apel D, Riemann JF. Palliation of nonresectable bile duct cancer: improved survival after photodynamic therapy. Am J Gastroenterol. 2005;100:2426–2430. doi: 10.1111/j.1572-0241.2005.00318.x. [DOI] [PubMed] [Google Scholar]
- 6.Ortner ME, Caca K, Berr F, et al. Successful photodynamic therapy for nonresectable cholangiocarcinoma: a randomized prospective study. Gastroenterology. 2003;125:1355–1363. doi: 10.1016/j.gastro.2003.07.015. [DOI] [PubMed] [Google Scholar]
- 7.Wong Kee Song LM, Wang KK, Zinsmeister AR. Mono-L-aspartyl chlorin e6 (NPe6) and hematoporphyrin derivative (HpD) in photodynamic therapy administered to a human cholangiocarcinoma model. Cancer. 1998;82:421–427. [PubMed] [Google Scholar]
- 8.Oertel M, Schastak SI, Tannapfel A, et al. Novel bacteriochlorine for high tissue-penetration: photodynamic properties in human biliary tract cancer cells in vitro and in a mouse tumour model. J Photochem Photobiol B. 2003;71:1–10. doi: 10.1016/s1011-1344(03)00091-5. [DOI] [PubMed] [Google Scholar]
- 9.de Groen PC, Gores GJ, LaRusso NF, Gunderson LL, Nagorney DM. Biliary tract cancers. N Engl J Med. 1999;341:1368–1378. doi: 10.1056/NEJM199910283411807. [DOI] [PubMed] [Google Scholar]
- 10.Gores GJ. Cholangiocarcinoma: current concepts and insights. Hepatology. 2003;37:961–969. doi: 10.1053/jhep.2003.50200. [DOI] [PubMed] [Google Scholar]
- 11.Weinbren K, Mutum SS. Pathological aspects of cholangiocarcinoma. J Pathol. 1983;139:217–238. doi: 10.1002/path.1711390210. [DOI] [PubMed] [Google Scholar]
- 12.Shimada H, Niimoto S, Matsuba A, Nakagawara G, Kobayashi M, Tsuchiya S. The infiltration of bile duct carcinoma along the bile duct wall. Int Surg. 1988;73:87–90. [PubMed] [Google Scholar]
- 13.Sakamoto E, Nimura Y, Hayakawa N, et al. The pattern of infiltration at the proximal border of hilar bile duct carcinoma: a histologic analysis of 62 resected cases. Ann Surg. 1998;227:405–411. doi: 10.1097/00000658-199803000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sako K, Seitzinger GL, Garside E. Carcinoma of the extrahepatic bile ducts: review of the literature and report of six cases. Surgery. 1957;41:416–437. [PubMed] [Google Scholar]
- 15.Gertsch P, Thomas P, Baer H, Lerut J, Zimmermann A, Blumgart LH. Multiple tumors of the biliary tract. Am J Surg. 1990;159:386–388. doi: 10.1016/s0002-9610(05)81278-4. [DOI] [PubMed] [Google Scholar]
- 16.Berr F, Tannapfel A, Lamesch P, et al. Neoadjuvant photodynamic therapy before curative resection of proximal bile duct carcinoma. J Hepatol. 2000;32:352–357. doi: 10.1016/s0168-8278(00)80083-5. [DOI] [PubMed] [Google Scholar]
- 17.Burke EC, Jarnagin WR, Hochwald SN, Pisters PW, Fong Y, Blumgart LH. Hilar cholangiocarcinoma: patterns of spread, the importance of hepatic resection for curative operation, and a presurgical clinical staging system. Ann Surg. 1998;228:385–394. doi: 10.1097/00000658-199809000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Polydorou AA, Cairns SR, Dowsett JF, et al. Palliation of proximal malignant biliary obstruction by endoscopic endoprosthesis insertion. Gut. 1991;32:685–689. doi: 10.1136/gut.32.6.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ducreux M, Liguory C, Lefebvre JF, et al. Management of malignant hilar biliary obstruction by endoscopy. Results and prognostic factors. Dig Dis Sci. 1992;37:778–783. doi: 10.1007/BF01296439. [DOI] [PubMed] [Google Scholar]
- 20.Chang WH, Kortan P, Haber GB. Outcome in patients with bifurcation tumors who undergo unilateral versus bilateral hepatic duct drainage. Gastrointest Endosc. 1998;47:354–362. doi: 10.1016/s0016-5107(98)70218-4. [DOI] [PubMed] [Google Scholar]
- 21.Davids PH, Groen AK, Rauws EA, Tytgat GN, Huibregtse K. Randomised trial of self-expanding metal stents versus polyethylene stents for distal malignant biliary obstruction. Lancet. 1992;340:1488–1492. doi: 10.1016/0140-6736(92)92752-2. [DOI] [PubMed] [Google Scholar]
- 22.Kaassis M, Boyer J, Dumas R, et al. Plastic or metal stents for malignant stricture of the common bile duct? Results of a randomized prospective study. Gastrointest Endosc. 2003;57:178–182. doi: 10.1067/mge.2003.66. [DOI] [PubMed] [Google Scholar]
- 23.Valle J, Wasan H, Palmer DH, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010;362:1273–1281. doi: 10.1056/NEJMoa0908721. [DOI] [PubMed] [Google Scholar]
- 24.Bowling TE, Galbraith SM, Hatfield AR, Solano J, Spittle MF. A retrospective comparison of endoscopic stenting alone with stenting and radiotherapy in non-resectable cholangiocarcinoma. Gut. 1996;39:852–855. doi: 10.1136/gut.39.6.852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Momm F, Schubert E, Henne K, et al. Stereotactic fractionated radiotherapy for Klatskin tumours. Radiother Oncol. 2010;95:99–102. doi: 10.1016/j.radonc.2010.03.013. [DOI] [PubMed] [Google Scholar]
- 26.Molt P, Hopfan S, Watson RC, Botet JF, Brennan MF. Intraluminal radiation therapy in the management of malignant biliary obstruction. Cancer. 1986;57:536–544. doi: 10.1002/1097-0142(19860201)57:3<536::aid-cncr2820570322>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 27.Cheon YK, Cho YD, Baek SH, et al. Comparison of survival of advanced hilar cholangiocarcinoma after biliary drainage alone versus photodynamic therapy with external drainage. Korean J Gastroenterol. 2004;44:280–287. [PubMed] [Google Scholar]
- 28.Wiedmann M, Caca K, Berr F, et al. Neoadjuvant photodynamic therapy as a new approach to treating hilar cholangiocarcinoma: a phase II pilot study. Cancer. 2003;97:2783–2790. doi: 10.1002/cncr.11401. [DOI] [PubMed] [Google Scholar]
- 29.Kiesslich T, Berlanda J, Plaetzer K, Krammer B, Berr F. Comparative characterization of the efficiency and cellular pharmacokinetics of Foscan- and Foslip-based photodynamic treatment in human biliary tract cancer cell lines. Photochem Photobiol Sci. 2007;6:619–627. doi: 10.1039/b617659c. [DOI] [PubMed] [Google Scholar]
- 30.McCaughan JS, Jr, Mertens BF, Cho C, Barabash RD, Payton HW. Photodynamic therapy to treat tumors of the extrahepatic biliary ducts. A case report. Arch Surg. 1991;126:111–113. doi: 10.1001/archsurg.1991.01410250119022. [DOI] [PubMed] [Google Scholar]
- 31.Ortner MA, Liebetruth J, Schreiber S, et al. Photodynamic therapy of nonresectable cholangiocarcinoma. Gastroenterology. 1998;114:536–542. doi: 10.1016/s0016-5085(98)70537-2. [DOI] [PubMed] [Google Scholar]
- 32.Tomizawa Y, Tian J. Photodynamic therapy for unresectable cholangiocarcinoma. Dig Dis Sci. 2012;57:274–283. doi: 10.1007/s10620-011-1957-7. [DOI] [PubMed] [Google Scholar]
- 33.Quyn AJ, Ziyaie D, Polignano FM, Tait IS. Photodynamic therapy is associated with an improvement in survival in patients with irresectable hilar cholangiocarcinoma. HPB (Oxford) 2009;11:570–577. doi: 10.1111/j.1477-2574.2009.00102.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee TY, Cheon YK, Shim CS, Cho YD. Photodynamic therapy prolongs metal stent patency in patients with unresectable hilar cholangiocarcinoma. World J Gastroenterol. 2012;18:5589–5594. doi: 10.3748/wjg.v18.i39.5589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nanashima A, Yamaguchi H, Shibasaki S, et al. Adjuvant photodynamic therapy for bile duct carcinoma after surgery: a preliminary study. J Gastroenterol. 2004;39:1095–1101. doi: 10.1007/s00535-004-1449-z. [DOI] [PubMed] [Google Scholar]
- 36.Berr F, Wiedmann M, Tannapfel A, et al. Photodynamic therapy for advanced bile duct cancer: evidence for improved palliation and extended survival. Hepatology. 2000;31:291–298. doi: 10.1002/hep.510310205. [DOI] [PubMed] [Google Scholar]
- 37.Shim CS, Cheon YK, Cha SW, et al. Prospective study of the effectiveness of percutaneous transhepatic photodynamic therapy for advanced bile duct cancer and the role of intraductal ultrasonography in response assessment. Endoscopy. 2005;37:425–433. doi: 10.1055/s-2005-861294. [DOI] [PubMed] [Google Scholar]
- 38.Utsumi K, Takai Y, Tada T, Ohzeki S, Fujiwara H, Hamaoka T. Enhanced production of IL-6 in tumor-bearing mice and determination of cells responsible for its augmented production. J Immunol. 1990;145:397–403. [PubMed] [Google Scholar]
- 39.Cheon YK, Cho YD, Moon JH, et al. Diagnostic utility of interleukin-6 (IL-6) for primary bile duct cancer and changes in serum IL-6 levels following photodynamic therapy. Am J Gastroenterol. 2007;102:2164–2170. doi: 10.1111/j.1572-0241.2007.01403.x. [DOI] [PubMed] [Google Scholar]
- 40.Goydos JS, Brumfield AM, Frezza E, Booth A, Lotze MT, Carty SE. Marked elevation of serum interleukin-6 in patients with cholangiocarcinoma: validation of utility as a clinical marker. Ann Surg. 1998;227:398–404. doi: 10.1097/00000658-199803000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Prasad GA, Wang KK, Baron TH, et al. Factors associated with increased survival after photodynamic therapy for cholangiocarcinoma. Clin Gastroenterol Hepatol. 2007;5:743–748. doi: 10.1016/j.cgh.2007.02.021. [DOI] [PubMed] [Google Scholar]
- 42.Cheon YK, Lee TY, Lee SM, Yoon JY, Shim CS. Longterm outcome of photodynamic therapy compared with biliary stenting alone in patients with advanced hilar cholangiocarcinoma. HPB (Oxford) 2012;14:185–193. doi: 10.1111/j.1477-2574.2011.00424.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dumoulin FL, Gerhardt T, Fuchs S, et al. Phase II study of photodynamic therapy and metal stent as palliative treatment for nonresectable hilar cholangiocarcinoma. Gastrointest Endosc. 2003;57:860–867. doi: 10.1016/s0016-5107(03)70021-2. [DOI] [PubMed] [Google Scholar]
- 44.Kahaleh M, Mishra R, Shami VM, et al. Unresectable cholangiocarcinoma: comparison of survival in biliary stenting alone versus stenting with photodynamic therapy. Clin Gastroenterol Hepatol. 2008;6:290–297. doi: 10.1016/j.cgh.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 45.Witzigmann H, Berr F, Ringel U, et al. Surgical and palliative management and outcome in 184 patients with hilar cholangiocarcinoma: palliative photodynamic therapy plus stenting is comparable to r1/r2 resection. Ann Surg. 2006;244:230–239. doi: 10.1097/01.sla.0000217639.10331.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wiedmann M, Berr F, Schiefke I, et al. Photodynamic therapy in patients with non-resectable hilar cholangiocarcinoma: 5-year follow-up of a prospective phase II study. Gastrointest Endosc. 2004;60:68–75. doi: 10.1016/s0016-5107(04)01288-x. [DOI] [PubMed] [Google Scholar]