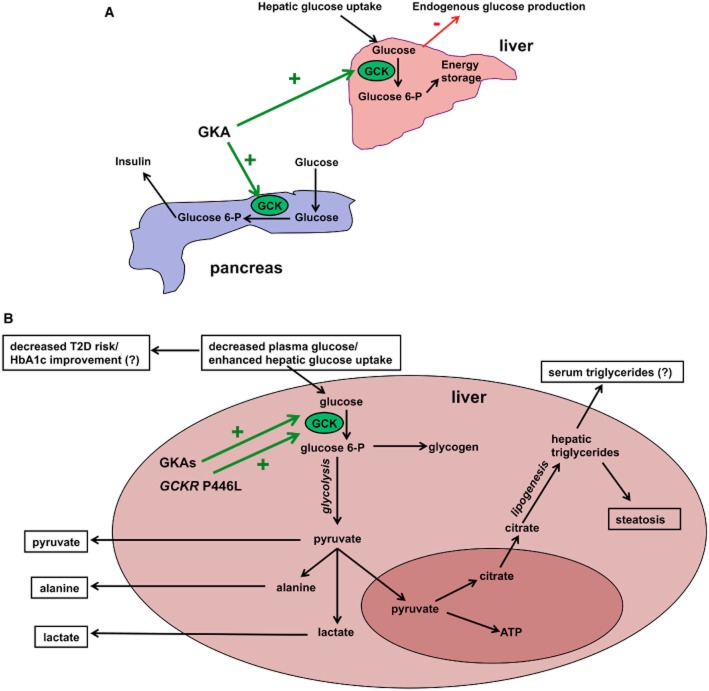
Figure 1.
Metabolic effects of GCK activation. (A) Schematic representation of the beneficial effects of GKAs on the pancreas and liver. GKAs lead to increased glucose-stimulated insulin secretion in the beta-cell and increase the glucose-6-phoshate : glucose ratio in the liver, resulting in enhanced hepatic glucose uptake and suppressed endogenous glucose production. (B) Potential shared hepatic pathways for seven phenotypic traits and metabolites significantly associated with both GKAs and the GCKR P446L variant. Activation of GCK by GKAs results in increased hepatic glucose uptake (Grimsby et al., 2003; De Ceuninck et al., 2012; Nissim et al., 2012), which likely underlies the association of P446L with plasma glucose concentrations (Dupuis et al., 2010). Decreased plasma glucose levels contribute to reduction of T2D risk, as seen with GCKR P446L (Dupuis et al., 2010), and/or decreased HbA1c, as observed with GKAs in rodent models and in short-term, but not long-term, human trials (Meininger et al., 2011; De Ceuninck et al., 2012). GKAs increase flux through glycolysis and hepatic lactate, pyruvate, and alanine output (Nissim et al., 2012). GCKR P446L is associated with plasma levels of all three glycolytic products (Suhre et al., 2011; Kettunen et al., 2012). However, enhanced glycolytic flux also stimulates de novo lipogenesis; both GKAs and P446L have been shown to increase hepatic steatosis (Speliotes et al., 2011; De Ceuninck et al., 2012), and P446L is associated with plasma triglycerides (Saxena et al., 2007). Emerging evidence suggests GKAs may also affect plasma triglycerides (Meininger et al., 2011; De Ceuninck et al., 2012). Significant published associations with both GCKR P446L and GKAs are shown in the black boxes, whilst contradictory findings for GKAs are indicated by a ‘?’.