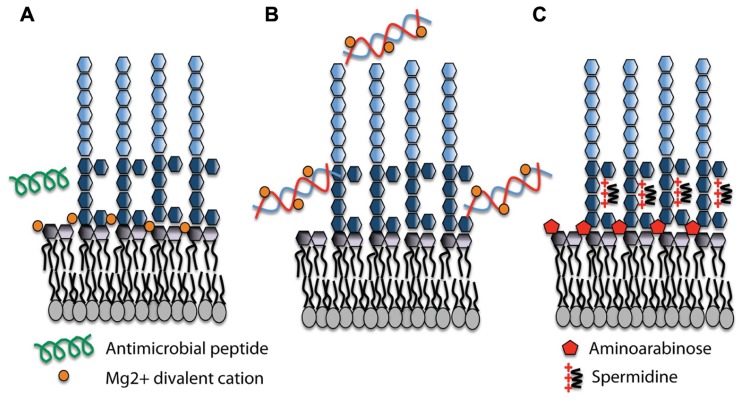
FIGURE 1.
Lipopolysaccharide (LPS) modifications in the presence of extracellular DNA that contribute to antimicrobial peptide resistance. (A) Divalent metal cations including Mg2+ (orange) bind to the negatively charged phosphates of the lipid A moiety of LPS and act to stabilize LPS. Antimicrobial peptides (green) can displace cations and disrupt membrane integrity, leading to cell lysis and death. (B) Extracellular DNA binds and sequesters cations from the environment and the membrane. (C) In response to limiting Mg2+ or cation chelation, the PhoPQ/PmrAB systems are activated leading to the production of covalently attached aminoarabinose to the phosphates of lipid A (red) and the production of polycation spermidine (charge, +3) on the surface, which may bind electrostatically to negative charges in the core oligosaccharide (dark blue) of the O antigen. Both modifications mask the negative charges and protect the outer membrane from peptide damage.