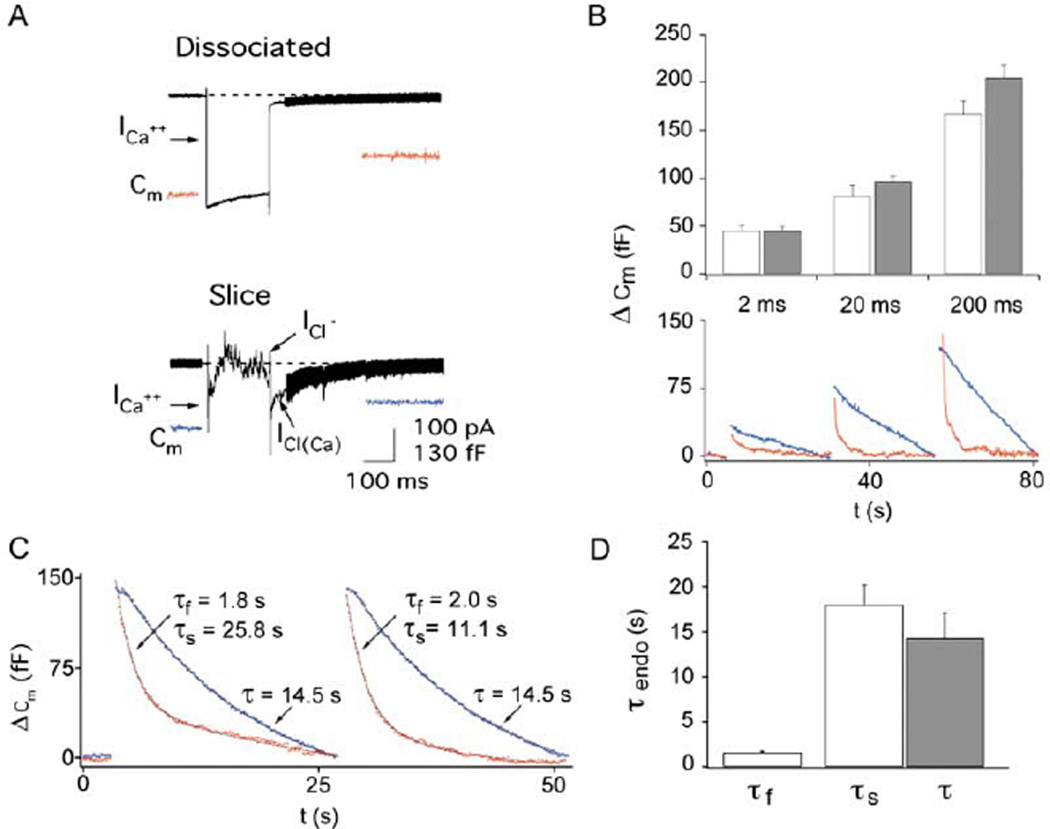
Figure 2. Exo-Endocytosis in Slice versus Acutely Dissociated Synaptic Terminals.
(A) Calcium currents in acutely dissociated terminals and isolated terminals in slice. Depolarizing the terminal in slice induces a ICa current and reciprocal GABAergic chloride feedback currents followed by a large inward Ca2+-activated chloride current [ICa(Cl)].
(B) Exocytosis (ΔCm) is the same in acutely dissociated terminals (open bars) and isolated terminals in the slice (gray bars) for depolarizations (−60 to 0 mV) of 2 ms (dissociated, n = 9; slice, n = 6), 20 ms (dissociated, n = 10; slice, n = 8), and 200 ms (dissociated, n = 23; slice, n = 35). Cm traces are from a typical cell in dissociation (red) and slice (blue) with consecutive 2, 20, and 200 ms depolarizations from −60 to 0 mV. Note that the percentage of fast endocytosis increases with pulse duration.
(C) Endocytosis is slower when recorded in slice (blue) as compared to dissociated terminals (red). Two successive 200 ms depolarizations each elicit endocytosis with both fast and slow kinetic components in acutely dissociated terminals, but only a single, slow kinetic component exists in terminals from the slice preparation.
(D) The average kinetics of endocytosis in the acute dissociation (open bars) were faster than those of the slice preparation (gray bars). The fast component of endocytosis in the acutely dissociated terminals is significantly different (p < 0.001) from the slow component of endocytosis in the same dissociated terminals (τslow = 18.0 ± 2.2 s; n = 23) as well as the single component of endocytosis from the slice preparation (τ = 14.3 ± 2.8 s; n = 26). Note that τslow in the dissociated terminals is not different from the τ of endocytosis in the slice (p = 0.32).