Fig. 5.
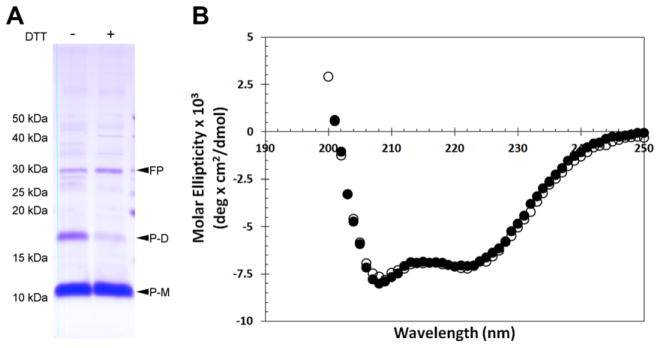
Circular dichroism spectroscopy of A2aR TM 7 indicates α-helical secondary structure. SDS–PAGE (16% acrylamide) shows that purified and dialyzed hA2aR TM 7 peptide forms dimers through a disulfide bond, where addition of DTT reduces these intermolecular bonds (A). Purified hA2aR TM 7 was diluted to 41.4 μM in 20mM phosphate buffer, pH 7.4, 0.1% Fos-choline 16 with (closed circles) and without (open circles) 5 mM TCEP and characterized by CD (B). Fusion protein (FP); monomeric hA2aR TM 7 peptide (P-M); putative dimeric hA2aR TM 7 peptide (P-D) are indicated with arrows.
