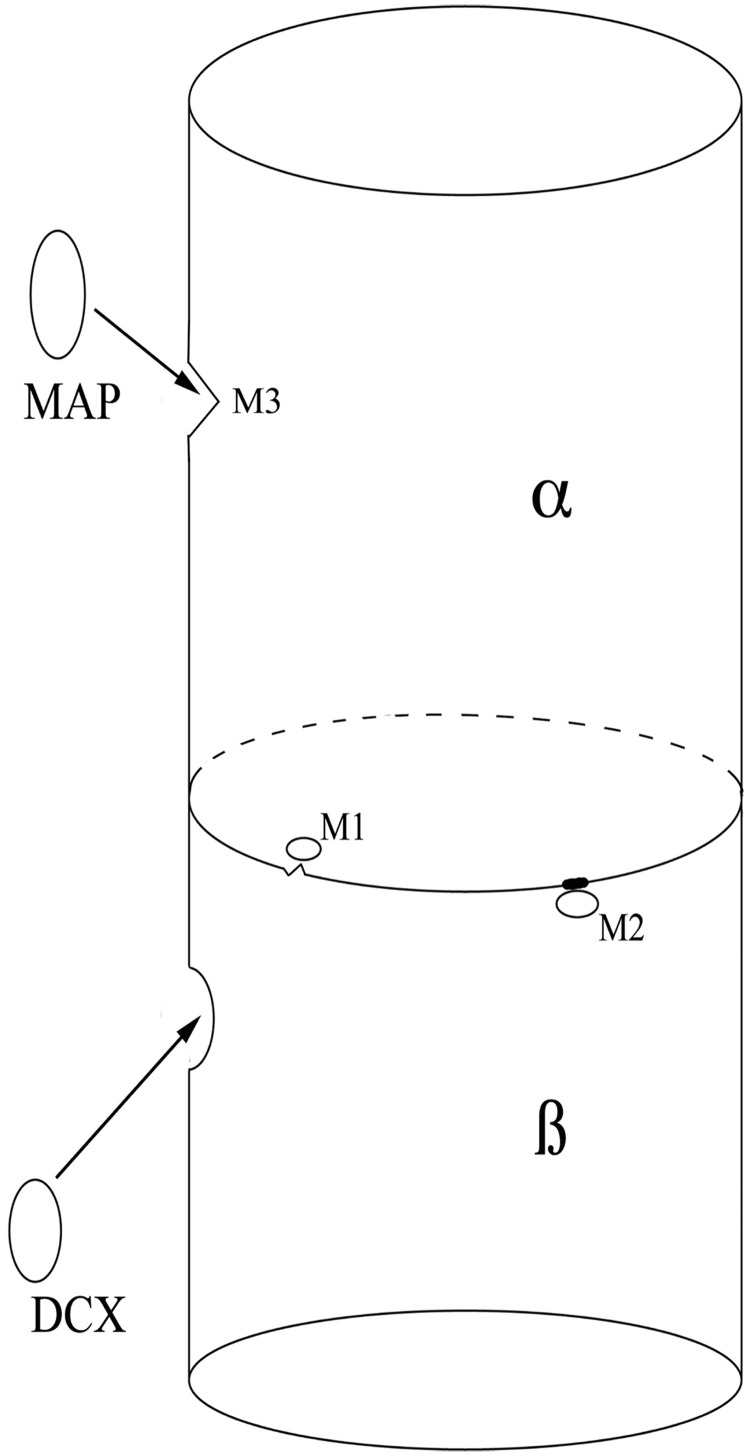
Figure 1.
Schematic showing the effects of mutations on the structure and stability of microtubules. This depicts a microtubule heterodimer, composed of alternating α- and β-subunits. Mutations near the interface of the subunits can interfere both with the dimerization process (owing to unstable regions of the interface that break down, M1) and rapid breakdown of the dimers (owing to impaired breakdown of abnormal regions of interface, M2) that are essential for neuronal migration and axonal pathfinding. This likely leads to hypoplastic axonal structures such as the cortical spinal tracts, corpus callosum and cranial nerves. Interaction with microtubule-associated proteins (MAPs) such as DCX (in β-subunit) is essential for other microtubule functions, such as mitosis and neuronal migration. Mutations near binding sites of the microtubule-associated proteins (M3 in α-subunit) can interfere with microtubule-associated protein binding, disturbing these critical functions in a manner similar to mutations of the microtubule-associated proteins themselves. These mutations are more likely to result in a malformation of cortical development.