Abstract
Genetic therapies for cystic fibrosis (CF) must be assessed for safety and efficacy, so testing in a non-human primate (NHP) model is invaluable. In this pilot study we determined if the conducting airways of marmosets (n = 2) could be transduced using an airway pre-treatment followed by an intratracheal bolus dose of a VSV-G pseudotyped HIV-1 based lentiviral (LV) vector (LacZ reporter). LacZ gene expression (X-gal) was assessed after 7 days and found primarily in conducting airway epithelia as well as in alveolar regions. The LacZ gene was not detected in liver or spleen via qPCR. Vector p24 protein bio-distribution into blood was transient. Dosing was well tolerated. This preliminary study confirmed the transducibility of CF-relevant airway cell types. The marmoset is a promising NHP model for testing and translating genetic treatments for CF airway disease towards clinical trials.
The relentless progression of cystic fibrosis (CF) airway disease remains an unresolved problem although the rate of decline has been slowed by the range of treatments now available. The majority of morbidity and mortality relates to the chronic airway infection and inflammation that commences in early infancy and leads to premature death from lung failure.
Gene-based correction of defective airway epithelial cell function, by introduction of the functional CF transmembrane conductance regulator (CFTR) gene, is a rational approach designed to produce lasting therapeutic benefit and could be the basis for a cure. Practical success would restore CFTR ion-channel function, producing a physiologically-balanced treatment for CF airway disease. Additionally, CFTR gene transduction of airway stem/progenitor cells should produce extended correction because the daughter cells that re-populate the airway epithelium contain the corrected CFTR gene1. Early correction, when CF lungs are considered to be functionally unaffected2, has the potential to prevent the initiation of CF lung disease.
In mice our single-dose lentiviral (LV) airway gene transfer technique produces immediate as well as long-term airway reporter gene transfer3, extending up to a mouse lifetime. Use of a lysophosphatidylcholine (LPC) airway pre-treatment improves gene transfer and may enable access to airway stem/progenitor cells able to support long term gene expression4. Importantly, this method has already shown its utility with the therapeutic CFTR gene; we have reported more than 12 months of functional CFTR gene expression in CF mouse airways5. Furthermore, we have demonstrated robust reporter gene transfer in mouse lung and produced low-level proof-of-principle expression in sheep lung6.
The aim of this pilot study was to test the potential of this method in the lungs of a non-human primate (NHP), the marmoset (Callithrix jacchus). The marmoset has a number of advantages as a NHP animal model for studying lung gene transfer. In particular, due to its small size, similar in body size to a very large rat, the modest LV volumes required are within the capabilities of current vector production methods.
Results
This preliminary study was designed to establish if our LV vector delivery system could transduce NHP conducting airway tissue prior to proposing and designing more extensive NHP gene transfer studies. For that reason in this short-term study only two animals (one male and one female) were used. No experimental controls were employed since published3,5,6,7 data and our previous studies in mice and sheep strongly indicate that none of these controls would elicit gene expression.
LPC and LV dose deliveries were uneventful. General behaviour (demeanour, feeding, respiration) and auscultation findings were within normal limits over the post-treatment study period. In both animals the gross appearance of the lungs was unremarkable when the chest was opened. Histological examination of lung tissue by a veterinary pathologist showed no abnormalities (including cellular infiltration) of consequence.
LacZ gene expression: Histology
One week post-dosing LacZ gene expression was visible in excised lung tissue from both marmosets, although the female showed a higher level of transduction (Figs. 1,2,3 from the female marmoset). Figure 1 shows an en face example of LacZ gene expression in lung tissue. LacZ gene expression was present primarily in surface and basal epithelial cells in the conducting airways. The patchy blue cell staining had a generally peribronchiolar distribution along conducting airways in several lobes (Fig. 1). In cross-sections of the trachea (Fig. 2) patchy transduction was observed across the full thickness of the epithelium.
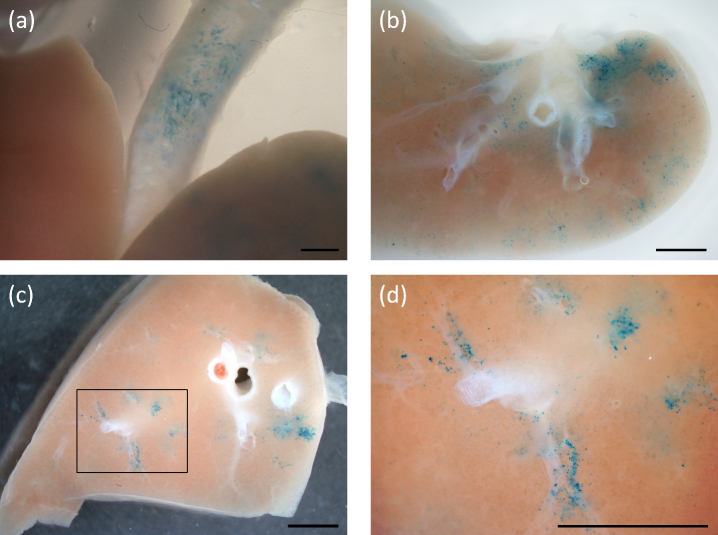
Figure 1. LacZ gene expression (blue stained cells) in marmoset lung one week after gene transfer.
Transduction across several lobes occurred in an airway-associated and a patchy-diffuse pattern. (a) Gene expression is apparent in the intact trachea above the carina and adjacent to the upper lobes seen here in the un-dissected lung after X-gal processing. (b) shows an en face gross lobe section with blue stained cell patches distributed around the lobe periphery. (c) In another thick en face section the patchy peri-bronchioar distribution is evident, with the boxed region enlarged for clarity in (d). Scale bars 2.5 mm.
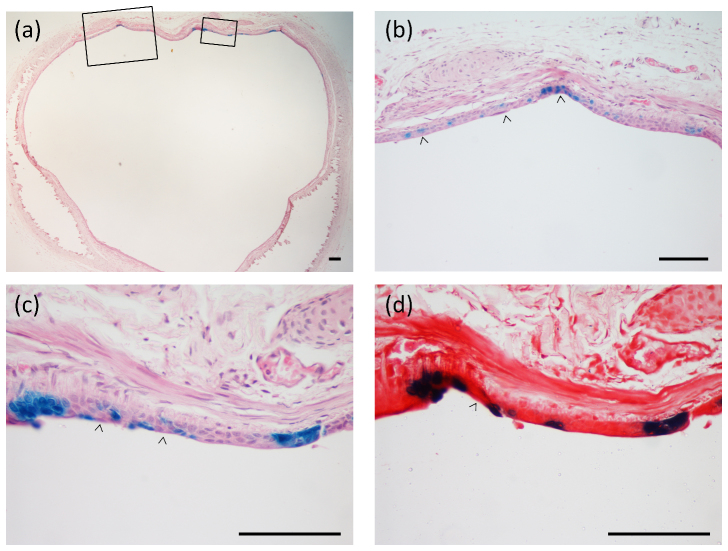
Figure 2.
(a) Cross-sections show regions of patchy LacZ gene expression along the trachea.The boxed areas in (a) are magnified in (b) and (c) to show the nature of transduction of the conducting airway epithelium. Examples of full thickness staining that includes basal cells (arrow heads), surface and individual-cell gene expression are shown. In (d) the next serial section adjacent to that shown in (c) is counterstained with Safranin-O and more clearly displays the epithelial layer staining. H&E stain in (a), (b), and (c). Scale bars 100 μm.
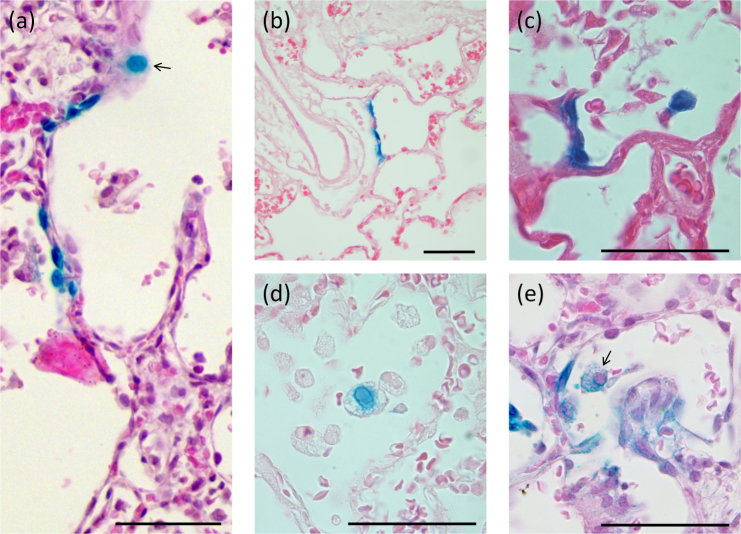
Figure 3. 7-day LacZ gene expression in the alveolar region.
(a) A grouping of LacZ-expressing alveolar cells are present together with an alveolar macrophage (arrow). Examples of (b) Type I pneumocyte transduction, and (c) Type II pneumocyte transduction of several adjacent cells with strong nuclear LacZ staining that has bled into the cytoplasm; (d) and (e) show examples of alveolar macrophage staining showing evidence of phagocytic LacZ staining (blue granules within the cytoplasm) and direct cellular transduction (blue nuclear staining; arrow), respectively. Scale bar 50 μm.
In the alveolar tissue (Fig. 3) there was substantially less cell transduction present compared to the conducting airways. The transduced cells in the distal lung airways included alveolar Type 1 and Type II cells (Fig. 3b–c) and inflammatory cells such as alveolar macrophages (Fig. 3d–e), and these were distributed across the lobes, again in a patchy pattern. The macrophages displayed blue LacZ staining indicating that both direct transduction and phagocytic capture of transduced cells could occur.
Histological analysis of the liver and spleen samples showed no LacZ gene expression.
LacZ gene presence: PCR
LacZ gene presence in the lung, liver and spleen was assessed by quantitative PCR (qPCR). The LacZ gene was detected in the lung tissue from the female marmoset (the ΔCt values for the three tissue samples, performed in triplicate, were 11.91, 6.57 and 5.89), but not in the sampled lung tissue from the male. The LacZ gene was not detected in the liver or spleen tissue from either marmoset, nor in scavenged (untreated colony cull) marmoset control tissue.
Vector particle dissemination
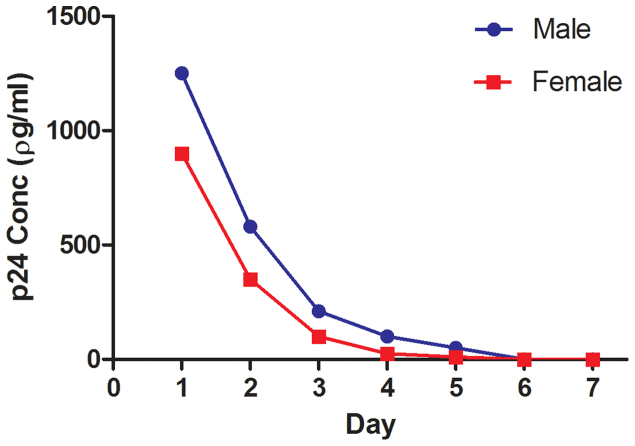
The protein p24 is a component of the HIV-based LV vector virion, and its presence in blood was used to determine the extent of non-target distribution of vector or vector constituents after vector delivery into the airway. The levels of serum p24 in both animals (Fig. 4) show that although the protein is found in serum one day after dosing the levels declined therafter and returned to baseline by day six.
Figure 4. p24 assay on marmoset serum demonstrates that the vector components are present in the blood shortly after dosing, but are cleared prior to day 7.
Discussion
To our knowledge these are the first studies to test and show that airway gene transfer can be achieved in vivo using a VSV-G pseudotyped HIV-based LV vector in an adult NHP model. Our findings extend earlier work in human airway cell cultures and tracheal explants showing efficient transduction with an Ebola-pseudotyped HIV vector8, and airway transduction in infant macaques after in utero parenchymal lung injection of a VSV-G pseudotyped HIV-based vector9. The primary finding was that the marmoset lung can be transduced using our LV vector delivery system. Based on our behavioural assessments, gross post mortem organ examination, and histological analysis, no adverse effects of either LPC or the LV vector were apparent. As this was a limited observational pilot-study design there were insufficient numbers of animals to provide detailed quantitative comparisons.
The patchy gene expression observed in both marmosets has also been observed in mice and sheep6 when fluid-based gene vector dosing was used. This may be caused by multiple factors including, but not limited to, incomplete coverage of the large surface area of the lung airways by the relatively small dose volume, specificity of the vector for particular cell types, and a mismatch in the areas treated by the LPC and LV vector doses. Importantly, the patchiness that would accompany LV-CFTR vector dosed in the same way does not prevent physiologically functional CFTR gene expression occurring in our CF mouse nasal airway studies3,5.
Ciliated airway surface epithelial cells are a primary target of CF gene therapies, but are terminally-differentiated10. Gene expression will be lost when those cells undergo apoptosis as part of normal lung cell turnover processes. The lifespan of these epithelial cells in the marmoset is unknown, but transduction of stem/progenitor cells may be important for producing long-term gene expression. Studies in mice suggest that the epithelial stem/progenitor cells responsible for regular regeneration of the conducting airway epithelium reside, in part, in the epithelial basal-cell compartment11. The transient permeabilisation that LPC imposes on epithelial cell tight junctions4 can provide access to those deep lying basal cells. Importantly, we detected LacZ gene expression in these deep lying epithelial cells at 7 days, suggesting our LV vector can reach regions where some niches of adult stem/progenitor cells are expected to reside in mammalian airways12. Similarly, alveolar epithelial cells and macrophages13 were also transduced, with the latter finding extending the results in rodents to a NHP model.
LacZ gene expression was not detected via X-gal staining or qPCR in any sub-epithelial tissues or in the liver or spleen. Although LacZ positive cells were visible in the lungs of both animals, the negative qPCR finding in the male may have been the result of the randomly selected tissue samples not containing any LacZ transduced cells. The finding of a transient presence of the vector protein component p24 in serum (Fig. 4) confirms that although LV vector or vector components can reach the circulation they are rapidly removed. Although even conventional lipid-based lung gene transfer events can deliver vector DNA to non-target organs14, further studies are required to elucidate the mechanisms and implications of any off-target organ transduction.
While the finding of gene transfer and expression in airway epithelial cells is clear, there are obvious limitations. The very small group size and absence of control animals (other than scavenged tissue for qPCR analyses) were deliberately chosen to prevent unwarranted use of additional NHPs should reporter gene transfer had not proven effective. With the success of this pilot study, future studies now warrant the use of larger group sizes including control animals. Studies should be designed to assess the longevity of gene expression, and should include a LV-only treatment group to directly determine the requirement for LPC in the production of both short and long-term lung gene expression. Additional detailed biosafety assays and physiological, behavioural, immunological and histological monitoring must also be included.
In summary, this pilot study demonstrated the successful extension of our LV delivery technique into a NHP lung. In the translation of basic and preclinical science into useful human therapies this initial success validates the use of LV gene delivery into a NHP lung and suggests marmosets could serve as a valuable pre-clinical model for translational studies in respiratory medicine.
Methods
This multi-institutional study was approved by the Animal Ethics committees of the Women's and Children's Health Network, South Australia; and Monash University, Victoria. Two marmosets, one female (F: ~275 g) and one male (M: ~300 g) were sourced from the marmoset colony of the NH&MRC National Primate Colony, Monash University. Animal husbandry, experiments and subsequent monitoring were performed under specialist NHP veterinarian supervision and care provided by Monash Animal Services, Victoria, Australia.
Airway pre-treatment
Airway pre-treatment with LPC (Sigma Aldrich L4129, prepared w/v in PBS) was given prior to a single airway gene transfer event. The LPC concentration (0.1% w/v) was derived from mouse studies5,6 where effectiveness and tolerability were previously established. LPC dose volumes (F: 200 μl and M: 350 μl) were calculated by scaling-up our successful mouse lung gene transfer doses6.
Gene vector
The nuclear-localised LacZ (NLS-LacZ) VSV-G pseudotyped HIV-1 based LV vector was a 5-plasmid non-replicating SIN vector produced according to previously published methods5,15. Virus titre was 1.2 × 109 viral genome equivalents per ml as assayed by quantitative PCR7. The LV vector volumes (F: 350 μl and M: 500 μl) were also estimated by scale-up from our mouse deliveries.
Pre-treatment and LV dosing
Anaesthetic induction was with 10 mg/ml alfaxalone (Jurox Rutherford, NSW, Australia) and animals were intubated with an endotracheal tube (Sheridan Uncuffed 2.0, Hudson RCI, USA) placed with the tip midway between the epiglottis and the carina. Anaesthesia was maintained using 1.25% – 1.5% isofluorane. Dosing was performed in a Class II biosafety cabinet with animals held supine throughout. The LV vector was delivered as a single fluid bolus to the trachea via the endotracheal tube over ten seconds, one hour after the LPC pre-treatment. Animals were held anaesthetised and supine in the cabinet for an additional hour after LV dosing before being extubated and placed in PC-2 caging facilities.
Monitoring
Vital signs were monitored throughout dosing and in the post-operative period. Body weight and general behaviour were monitored daily along with blood sampling (centrifuged at 13,000 rpm, with sera stored at −80°C until required).
Tissue harvesting
Animals were humanely killed at 7 days using an Alfaxalone induction followed by pentobarbital overdose. The lungs were inflation-fixed in situ in 2% paraformaldehyde/0.5% glutaraldehyde (PFA/Glut) in PBS at 4°C and a pressure of 30 cmH2O for 15 mins. Samples of liver and spleen were also harvested and fixed in PFA/Glut at 4°C.
LacZ gene expression: Histology
The lungs were then excised and submerged in fresh chilled PFA/Glut overnight, and were subsequently processed for LacZ expression by our standard X-gal staining method6. The extent of LacZ gene expression in airway cells was first assessed en face in gross transversely-sectioned portions of lung. Blocks that showed blue LacZ staining were then prepared for routine histological sectioning with haematoxylin/eosin (H&E) or a Safranin-O counterstain.
LacZ gene presence: qPCR
Triplicate tissue samples (20 mg) were selected from random locations in the lungs, liver and spleen. Scavenged tissue from untreated animals was used as a negative control. All lung samples were then incubated at 55°C overnight in tail lysis buffer (Viagen Biotech, USA, Cat. # AB102-T) and 20 mg/ml Proteinase K (Promega, USA, Cat. # V3021) at a 1:50 ratio then heated to 80°C for one hour and crude lysates were stored at −20°C until analysis. All other samples were processed via the Wizard SV Genomic DNA Purification System (Promega, USA, Cat. # A2361) according to manufacturers specifications.
qPCR for the NLS-LacZ gene and marmoset GAPDH housekeeping gene16 was performed in a 96 well plate format (CFX Connect Real-Time PCR, Bio-Rad) according to the manufacturers standard protocol. Specific amplification was detected using SYBR Green (Fast SYBR Green Master Mix, Applied Biosystems USA, Cat. # 4381562). Cycle thresholds (Ct) for the LacZ gene were normalised with respect to the housekeeping gene and presented as ΔCt. The following primers were used: NLS-LacZ forward GCC ACT TCT TGA TGG ACC ACT T, NLS-LacZ reverse CCG CCA CCG ACA TCA TCT, GAPDH forward AAA GTG GAT GTC GTC GCC ATC AAT GAT and GAPDH reverse CTG GAA GAT GGT GAT GGG ATT TCC ATT (GeneWorks, Australia).
Vector particle dissemination
Sera were analysed using a HIV-1 p24 ELISA Kit (Perkin Elmer Life Sciences USA Ca# NEK050) performed as per manufacturer instructions.
Author Contributions
D.W.P. conceived and designed the study. D.W.P., D.M. and R.B. performed the gene transfer protocols; N.F., D.M., R.B. and D.W.P. completed tissue harvesting; N.F., D.M., P.C., R.B., M.D. and D.W.P. conducted laboratory studies and data analyses; D.W.P. and M.D. wrote the manuscript. All authors reviewed the manuscript.
Acknowledgments
Studies were supported by an NHMRC project grant, with supplemental assistance from the Cure4CF Foundation Ltd. Research veterinarians Dr Anne Gibbon and Dr Chris Mackay provided expert handling, intubation, anaesthesia, post-operative and ongoing husbandry for the marmosets. Ruth Williams (Adelaide Microscopy) performed sectioning and staining. Dr John Finnie (SA Pathology) provided histological assessment of lung sections. A/Prof Don Anson provided the LV vector. Shannon LeBlanc (Centre for Stem Cell Research) assisted with qPCR analysis. NF was supported by the MS McLeod PhD scholarship and CF Australia.
References
- Parsons D. W. Airway gene therapy and cystic fibrosis. J Paediatr Child Health 41, 94–96 (2005). [DOI] [PubMed] [Google Scholar]
- Stick S. M. & Sly P. D. Exciting new clinical trials in cystic fibrosis: infants need not apply. American journal of respiratory and critical care medicine 183, 1577–1578 (2011). [DOI] [PubMed] [Google Scholar]
- Limberis M., Anson D. S., Fuller M. & Parsons D. W. Recovery of airway cystic fibrosis transmembrane conductance regulator function in mice with cystic fibrosis after single-dose lentivirus-mediated gene transfer. Human gene therapy 13, 1961–1970 (2002). [DOI] [PubMed] [Google Scholar]
- Cmielewski P., Anson D. S. & Parsons D. W. Lysophosphatidylcholine as an adjuvant for lentiviral vector mediated gene transfer to airway epithelium: effect of acyl chain length. Respir Res 11, 84 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocker A. G. et al. Single-dose lentiviral gene transfer for lifetime airway gene expression. The journal of gene medicine 11, 861–867 (2009). [DOI] [PubMed] [Google Scholar]
- Liu C. et al. Lentiviral airway gene transfer in lungs of mice and sheep: successes and challenges. The journal of gene medicine 12, 647–658 (2010). [DOI] [PubMed] [Google Scholar]
- Kremer K. L., Dunning K. R., Parsons D. W. & Anson D. S. Gene delivery to airway epithelial cells in vivo: a direct comparison of apical and basolateral transduction strategies using pseudotyped lentivirus vectors. The journal of gene medicine 9, 362–368 (2007). [DOI] [PubMed] [Google Scholar]
- Kobinger G. P., Weiner D. J., Yu Q. C. & Wilson J. M. Filovirus-pseudotyped lentiviral vector can efficiently and stably transduce airway epithelia in vivo. Nature biotechnology 19, 225–230 (2001). [DOI] [PubMed] [Google Scholar]
- Tarantal A. F. et al. Lentiviral vector gene transfer into fetal rhesus monkeys (Macaca mulatta): lung-targeting approaches. Molecular therapy : the journal of the American Society of Gene Therapy 4, 614–621 (2001). [DOI] [PubMed] [Google Scholar]
- Rawlins E. L. & Hogan B. L. Ciliated epithelial cell lifespan in the mouse trachea and lung. Am J Physiol Lung Cell Mol Physiol 295, L231–234 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock J. R., Randell S. H. & Hogan B. L. Airway basal stem cells: a perspective on their roles in epithelial homeostasis and remodeling. Disease models & mechanisms 3, 545–556 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock J. R. et al. Basal cells as stem cells of the mouse trachea and human airway epithelium. Proceedings of the National Academy of Sciences of the United States of America 106, 12771–12775 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson A. A. et al. Amelioration of emphysema in mice through lentiviral transduction of long-lived pulmonary alveolar macrophages. J Clin Invest 120, 379–389 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyde S. et al. Multiple Doses Of Lipid-Mediated Gene Therapy Nebulised To The Mouse Lung Show Robust And Sustained Cftr Expression. Pediatric Pulmonology 46, 281 (2011). [Google Scholar]
- Koldej R., Cmielewski P., Stocker A., Parsons D. W. & Anson D. S. Optimisation of a multipartite human immunodeficiency virus based vector system; control of virus infectivity and large-scale production. The journal of gene medicine 7, 1390–1399 (2005). [DOI] [PubMed] [Google Scholar]
- Lundwall A., Larne O., Nayudu P. L., Ceder Y. & Valtonen-Andre C. Rapidly evolving marmoset MSMB genes are differently expressed in the male genital tract. Reprod Biol Endocrinol 7, 96 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]