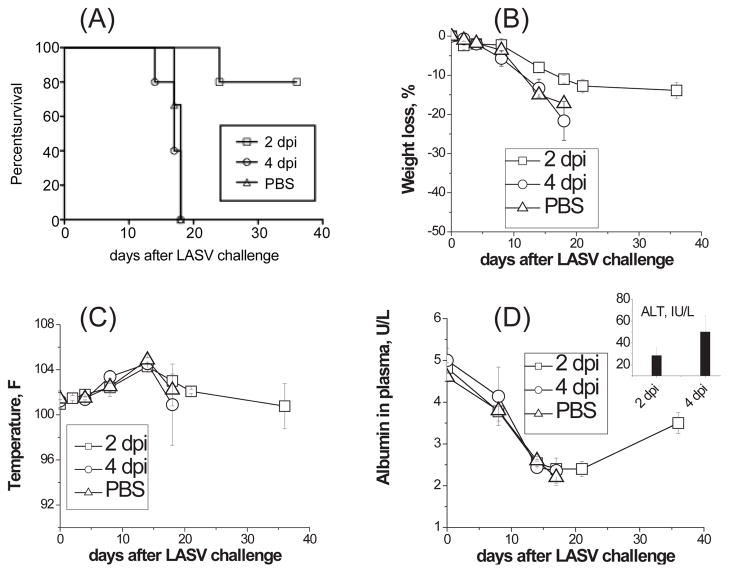
Figure 1. Post-challenge ML29 vaccination.
Two groups of strain 13 guinea pigs (5 animals/group) were challenged with LASV-Josiah (1000 PFU) and 2 and 4 days after the challenge animals received ML29 (1,000 PFU). Control groups received PBS (challenge control). Survival (A), weight loss (B), temperature (C), plasma levels of albumin (D) and ALT (insertion in panel D) were monitored. Survived animals were necropsied at the end of the experiment, on day 36 after LASV challenge.