Figure 2. Viral RNA load in tissues.
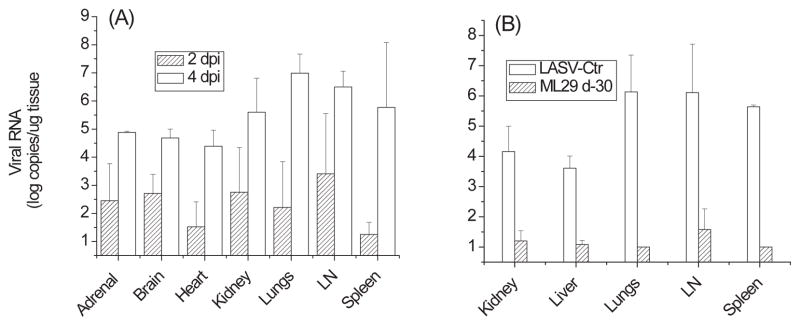
Tissue samples from animal groups described in figure 1 and from a vaccine control group (immunization with ML29 and challenge on day 30 with LASV, see Materials and Methods) were homogenized using the TissueLyser (Qiagen) RNA was extracted following the Trizol Reagent protocol (Invitrogen). Real-time qRT-PCR was performed using an ABI PRISM 7700 Sequence Detection System (Applied Biosystems, Foster City, CA) and RNA Ultrasense one-step real-time qRT-PCR system (Invitrogen) according to manufacturer’s recommendations. The primer/probe set for LASV-Josiah RNA targeted a GPC region of the S segment using LASV 36E2 and 80F2 primers [66]. Standards used in qRT-PCR were generated from serial 10-fold dilutions of RNA isolated from LASV-Josiah stock (10−1 PFU/ml – 10−7 PFU/ml) that were enumerated in triplicate by conventional plaque assay as previously described [12]. Sensitivity of qRT-PCR was 100 PFU-equivalent per ml of blood or per g of tissue.
