Abstract
Tropical endomyocardial fibrosis in India was a common medical problem in the coastal districts of south India, especially the Kerala State. The clinical and autopsy studies have shown left and right ventricular apical fibrosis, with varying degree of atrioventricular valve regurgitation. Left ventricular endomyocardial fibrosis presents with severe pulmonary hypertension and right ventricular endomyocardial fibrosis presents very high systemic venous pressure and congestive cardiac failure. Surgical management improved the natural history of the disease to some extent. Various infectious and toxic factors were postulated regarding its aetiology. During the last few years, incidence of the disease has decreased considerably. The only explanation identified is the significant improvement in the living standards of the people with the corresponding decline in the childhood malnutrition, infections, worm infestation and associated eosinophilia.
Keywords: Endocardial calcification, eosinophils, geographic pathology, malnutrition, restrictive cardiomyopathy
Introduction
The epidemiology of endomyocardial disease, is a ‘vanishing mystery’ in the southern districts of India especially in the coastal belt of Kerala state, which was once ‘the hot spot’ for this enigmatic disease1. On the other hand, the disease continues as the commonest cause of restrictive cardiomyopathy in Africa where it is a public health problem, widely discussed as a neglected tropical disease2. In a recent rural community survey in Mozambique, 20 per cent of the people studied had echocardiographic evidence for the disease with a male preponderance3. The disease affected children and young adults in an epidemic fashion with a geographical distribution (Fig. 1) within 15 degrees on either side of the equator4. Historical aspects of this disease leave a bright reflection on the geographical pathology and scientific enquiry on to the causation of an enigmatic disorder. September 2012 has marked the 65th year of the initial description of the disease and this centenary review gives an opportunity to outline the multi-disciplinary research that happened in India5.
Fig. 1.
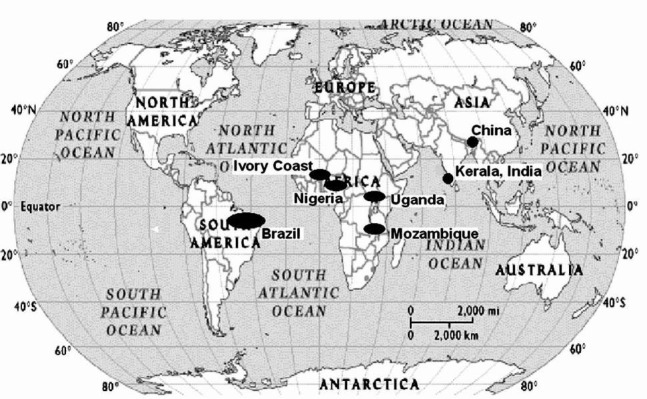
Geographic distribution of endemic regions of endomyocardial fibrosis. Reprinted with permission from BMJ Publishing Group Ltd.[Heart2009; 95: 9-14]1.
JNP Davies first coined the term endomyocardial fibrosis (EMF) while working in Uganda and said that “he became convinced rightly or wrongly that he had met a new disease”6. He also discussed the initial clinical description of the disease by Bedford and Constam in 19467. In fact, in 1938, Arthur Williams, the foundation professor of medicine at Makerere University, Kampala, Uganda had described two cases of mitral incompetence and correlated with large patches of fibrosis affecting the ventricular walls at necropsy and this is perhaps the earliest documentation of EMF in literature. Davies joined the same hospital in 1946 and did his MD thesis on this disease where he described the pathological features as a distinct entity. At the Royal Society meeting in 1954 he described the classical four pathological features of EMF and its distribution in Africa8. The typical features described were right and left ventricular endocardial fibrosis, affecting the apex and inflow region with atrioventricular valve regurgitation. To honour his seminal contribution, the disease came to be known as the Davies’ disease9,10. At the Mulago hospital in Uganda, where he worked, this disease accounted for 15 per cent of the deaths due to congestive cardiac failure9. Bedford, who visited the University in 1948, encouraged him to publish his observations and Arthur Williams and JD Ball were his co-authors. Later, JD Ball, with his missionary job joined Christian Medial College Vellore in India and identified the pathological specimens at autopsy which he shipped to Davies for confirmation. Thus, the disease was reported from India for the first time.
Samuel and Anklesaria published this initial autopsy series from south India in 196011,12. CK Gopi, in 1962, from Trivandrum, described the specimen kept in the hospital autopsied in 1950s as a case of right ventricular endomyocardial fibrosis with right atrial thrombi12. This was published earlier as a case of “Ball valve thrombus in the right atrium”. The initial pathological reports were followed by the description of clinical and angiographic features13,14. The triad of elevated jugular venous pressure, ascites and hepatomegaly formed the hallmark of right ventricular EMF, which can present with cyanosis and clubbing because of stretch opening of foramen ovale15,16. Mild cardiomegaly, loud left ventricular third heart sound, short systolic murmur and severe pulmonary hypertension formed the hallmark for the diagnosis of left ventricular endomyocardial fibrosis11. Routine laboratory evaluations are rarely abnormal, and a variety of other investigatory features were utilized for the diagnosis17–23. Chest X-rays show varying degree of cardiomegaly and at times typical endocardial calcification23. Fig. 2 summarizes the radiologic spectrum in endomyocardial fibrosis.
Fig. 2.
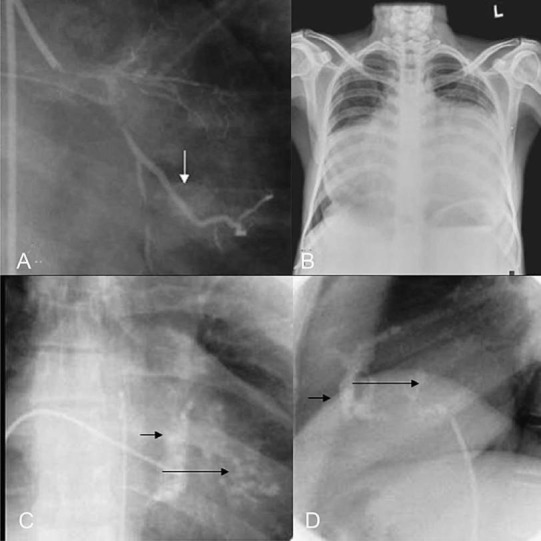
The radiologic spectrum in endomyocardial fibrosis. (A) Incidental detection of endocardial calcium during coronary angiogram, (B) Massive cardiomegaly in a patient with congestive cardiac failure. Lower panel shows the fluoroscopic images of endocardial calcium in frontal (C) and lateral view (D), short arrows show, right ventricular and long arrows show left ventricular endocardial calcium. Reprinted with permission from BMJ Publishing Group Ltd. [Heart2009; 95: 9-14]1.
Electrocardiogram shows low voltages, some features of ventricular hypertrophy with intra- ventricular conduction defects and non specific ST T changes20. Right ventricular EMF with severe right atrial dilatation is reflected in the electrocardiogram as qR pattern in V1. Evolution of two dimensional and colour Doppler echocardiography identified the typical Doppler and two dimensional features like atrial dilatation, spontaneous intracavitary echos which preceded the formation of thrombi17. The atrioventricular valve abnormalities, right and left ventricular apical fibrosis detected by 2-dimensional echocardiography made the diagnosis of this disease very easy. Attempts were made to characterize the tissue texture variations of fibrosis by colour coding18. Today echocardiography is used as the screening tool at the community level as the diagnosis of EMF could be confirmed at the bedside. Advanced imaging techniques like magnetic resonance imaging (MRI) and haemodynamic studies are done only for research purposes3,19. Two dimensional echocardiographic feature of left ventricular endomyocardial fibrosis is illustrated in Fig. 3.
Fig. 3.
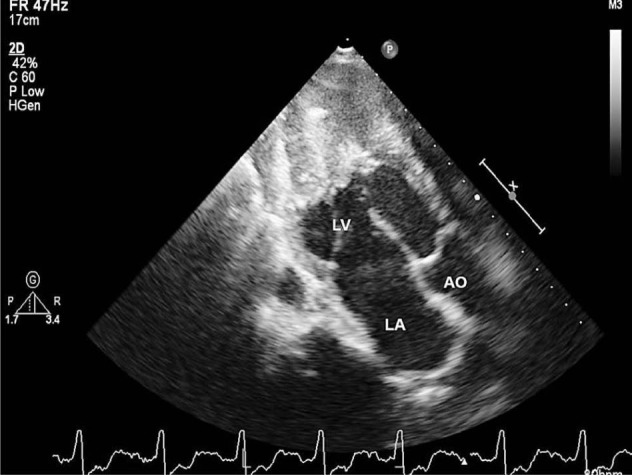
Two dimensional echocardiogram of left ventricular endomyocardial fibrosis showing dense calcified fibrous tissue obliterating the cavity of the ventricular apex. LV- left ventricle, LA - left atrium and AO - aorta.
The typical haemodynamic finding on cardiac catheterization is the dip and plateau pattern of restrictive ventricular filling, which does not show pressure equalization between ventricles, unlike constrictive pericarditis. Fig. 4 illustrates the typical angio-cardiographic features of endomyocardial fibrosis11,14.
Fig. 4.
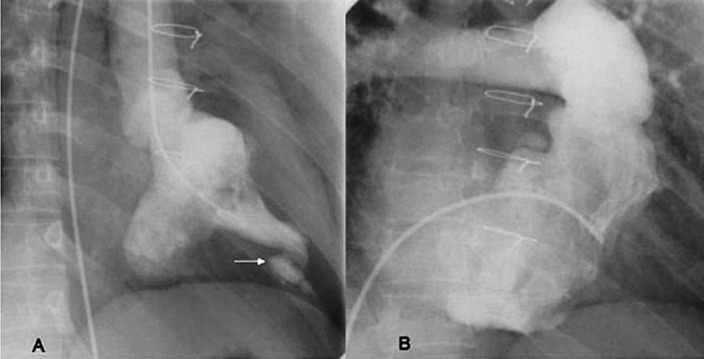
Angiocardiographic features of typical endomyocardial fibrosis showing apical ventricular cavity obliteration. (A) left ventricular angiogram in right anterior oblique view and the arrow points to an apical diverticuation, and (B) shows right ventricular angiogram in shallow right anterior oblique view. Reprinted with permission from BMJ Publishing Group Ltd. [Heart2009; 95: 9-14]1.
Pathology
Sub-endocardial fibrosis affecting the apices and the inflow tract of the right or left ventricle, or both, defines the disease8. This restrictive scarring prevents ventricular filling, generating a loud third heart sound. Fibrosis of the papillary muscles produces atrioventricular valve regurgitation, which aggravate the progressive atrial dilatation and leads to atrial fibrillation1. Tricuspid regurgitation is the mildest form, since plastering of the posterior tricuspid valve is better tolerated in the pulmonary circulation8. Calcification, an impressive finding on imaging denotes a burned out phase. Like the peculiar geographical distribution, the fibrotic endomyocardial involvement stops short of the ventricular outflow tract like a ridge24. Though atrio-ventricular valve regurgitation is typical of the disease, isolated right ventricular inflow obstruction and right ventricular outflow tract obstruction have been observed in isolated reports25,26.
At gross examination, the ventricles look small with grossly dilated atria, and thrombi are usually seen in the atria denoting stagnation and atrial fibrillation9,10. The pericardium looks normal but pericardial effusion and ascites are common findings27,28. The fibrotic retraction of the right ventricular apex produces the typical apical dimple29 which is demonstrable by 2-dimensional echocardiography. The fibrotic thickened endocardium can be decorticated as a separate layer from the rest of the myocardium, which forms the basis of the surgical procedure of endocardiectomy1. Often, the damage to the chordo-papillary structures warrants atrioventricular valve replacement. Microscopic and ultramicroscopic studies have shown a superficial layer formed by the fibrotic tissue rich in mucopolysaccharides, and also a deeper layer, which has lymphocytic infiltration with dilated lymphatics and damaged myocytes. The myocytes in the periphery are hypertrophied with normal epicardial coronary vessels and microvasculature29,30.
Clinical course
The early part of the disease is rarely clinically recognized in India and the disease comes to attention in the late stages and isolated endocardial involvement and intracardiac thrombi are the peculiar features1. Davies described three phases of the disease in his patients from Uganda. The initial phase is an acute carditis phase, characterized by febrile illness and in severe cases with heart failure and shock9. Those who survive this acute illness, progress into a sub acute phase followed by a chronic phase. Most of the patients come to clinical attention in this chronic burnt-out phase. Because of the abbreviated natural history, most authors have not identified progression of the disease in the other ventricle when one of the ventricles alone is involved. Though surgical series from the west has noted progression of the disease, data from our centre has not documented the progression of the disorder20,29. Once clinically diagnosed, the onset of complications like atrial fibrillation, thrombo-embolism, and progressive atrioventricular valve regurgitation abbreviates the natural history29. This is one of the rare disorders where spontaneous evolution of Fontan physiology is illustrated31. Fontan physiology is characterized by elevation of the mean systemic venous pressures above the diastolic pulmonary artery pressures. Because of this documented increase in systemic venous pressures above the pulmonary artery diastolic pressures, initial reports of right ventricular endomyocardial fibrosis were discussed as “isolated systemic venous hypertension”. The classical features of this isolated systemic venous hypertension include dilated hepatic veins, which show minimal inspiratory collapse, significant elevation of jugular venous pressure with prominent atrial waves, stretching open of the foramen ovale, which leads to right to left shunting and central cyanosis with clubbing15. The tricuspid valve is essentially normal in structure and function and the impaired filling of the ventricle with the augmented atrial contraction leads to diastolic opening of the pulmonary valve demonstrable by colour Doppler, pulsed Doppler and intracardiac pressure recording14. With the onset of atrial fibrillation, the right sided chambers act as a passive conduit, the forward flow essentially is now maintained by the elevated systemic venous pressure and intrathoracic pressure changes of respiration as in surgically created Fontan circulation31. Pericardial effusion and ascites dominates the clinical picture of right heart failure27. Ascites in endomyocardial fibrosis is out of proportion to pedal oedema as the cardiac index is maintained for a long time. However, the very high venous pressure results in high protein content in the pericardial and ascitic fluid. Why pleura are spared in these effusions is yet another peculiarity noted. Fig 5 shows the clinical and echocardiographic features of isolated right ventricular fibrosis.
Fig. 5.
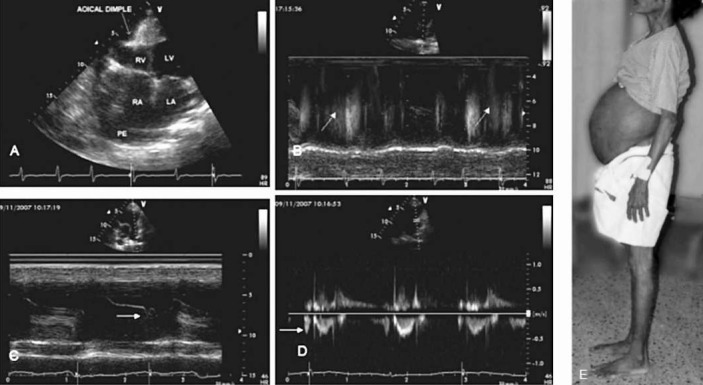
The clinical and echocardiographic features of right ventricular endomyocardial fibrosis. Clinical picture (E) shows the massive ascites with no pedal oedema. Echo pictures A to D show: A. apical 4 chamber view showing the fibrotic obliteration of the right ventricle (RV), with apical dimpling, grossly dilated right atrium (RA). B. M mode colour Doppler, of the pulmonary artery showing atrial systolic forward flow into pulmonary artery. C. M Mode echo showing atrial systolic opening of the pulmonary valve. D. Colour Doppler M mode echocardiogram showing the diastolic forward flow into pulmonary artery. Reprinted with permission from BMJ Publishing Group Ltd. [Heart2009; 95: 9-14]1.
Geographic pathology and associations
Historically, one of the most intriguing aspects of the disease is its peculiar occurrence in certain pockets of the world4. The disease is most common in the tropics 15° on either side of the equator5. Isolated case reports have been published from various parts of the world but many of these patients had long stay in the regions of endemicity. The initial description from Africa was followed by similar reports from Venezuela, Colombia and Brazil. Reports from Ceylon and India completed the “geographical belt” which mimicked that of Burkitt's lymphoma32. Subsequent studies identified Kerala as the hot spot for the disease with isolated reports from rest of India as well33. In Kerala during late 1970s and 1980s, 2.5 per cent of all cardiac admissions below the age of 40 years were contributed by patients with EMF29. In 1980 alone Trivandrum medical college registered 36 new patients with endomyocardial fibrosis, which was one of the highest reported numbers from India34. During the same period, >20 per cent of the patients below the age of 40 years, who got admitted with heart failure in the Ivory Coast, were diagnosed to have this disorder29. Many reports from Britain and other temperate countries have appeared in the literature, but those affected were migrants from tropical countries or in those native Europeans who resided in tropical countries for a long period29. This geographic localization to specific coastal belt and involvement of people in the lower socio-economic group intensified the research efforts on specific issues that can be postulated as the possible aetiology of this disease35,36. These efforts established international collaboration to compare and contrast the clinical features of similar rare disorders like Loffler's endocarditis and to define the role of nutrition, immunologic factors and eosinophils in the causation of EMF37,38 (Fig. 6).
Fig. 6.
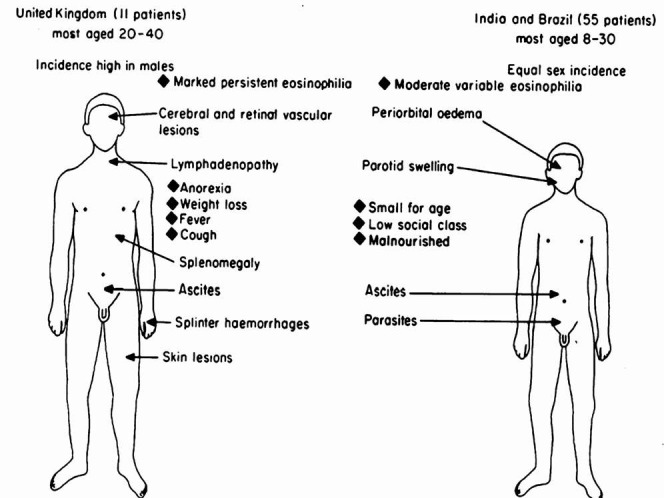
Summarizes the clinical features of the subjects seen with endomyocardial fibrosis in the tropics and the United Kingdom as reported in 198337. Reprinted with permission from bmj.com [Postgrad Med J1983; 59: 179-85].
Initial reports suggested some association with malaria, toxoplasma infection, rheumatic fever, chronic beriberi, loa loa infection, and lymphatic obstruction due to filariasis26. Indian Council of Medical Research took the lead and encouraged the research activities in India. These efforts culminated in two international symposia dedicated to understanding of this disorder34,39. Nutrition, infection and toxic factors were the main topics of discussion regarding its aetiolgy16,29,36. Establishment of local registries further defined the natural history and the outcomes of attempted surgical palliation40. Attempts were made to generate animal models13,41.
Reports of eosinophilic heart disease from the non tropics suggested that endocardial fibrosis could be the final result of a circulating toxic factor42. Brockington and Olsen collected the specimens from various centers and established that the final stages of endemic endomyocardial fibrosis and sporadic Loffler's endocardial disease are indistinguishable43. Eosinophils formed the centre stage for study during the 1980s. Clinical differences between the endemic disease in Kerala and sporadic disease in the West were discussed29,37. The tropical region with its heavy load of filarial infection and worm infestation had eosinophilia as a common disorder in childhood, which was postulated to initiate the disease in susceptible children. The systemic phase remained unrecognized and the patient came to clinical attention during the burnt-out phase. In contrast, non tropical eosinophilic syndrome affected people of better socio-economic category with male preponderance. The acute phase of eosinophilia is characterized by a systemic phase with thromboembolic complication, which later evolved into the fibrotic phase in survivors, indistinguishable from endomyocardial fibrosis44. The descriptions of coexisting rheumatic heart disease, aorto-arteritis, endocarditis, connective tissue diseases and occurrence in siblings were sporadic45,46. Eosinophilic origin of this disorder could not be proven by epidemiologic studies47.
The cyanogenic glycosides in tapioca, and Argemone mexicana, high vitamin D and cerium content of tapioca, serotonin in plantain were the toxic agents evaluated29,36,48. Induced protein deficiency with dietary excess of carbohydrates provided by banana and tapioca rich diets were used to induce animal models41,48–50. Spontaneous cardiomyopathy similar to endomyocardial fibrosis is reported in cats, whereas animal models were generated in dogs, monkeys and rats. The toxic theory got further support when patients on treatment with adriamycin and methysergide developed similar endocardial thickening29. Potassium deficiency because of repeated episodes of diarrhoea; collagenosis of connective tissue disorders, cirrhosis and infections were considered because of the temporal association in some patients but could not be substantiated8,51. Isolated right ventricular involvement in carcinoid heart disease generated lot of interest to identify a dietary toxin, similar to serotonin52. Excess of plantain consumption, which was high in serotonin but low in protein, was observed in this endemic population and therefore, was investigated in great detail from a causative stand point53. But none of these causes postulated could establish itself as the reason for the enigmatic disease54. Protein deficiency during childhood and during pregnancy, is postulated for the bimodal distribution of the disease in women55. Certain pockets in Uganda and Nigeria in Africa, and Kerala in south India, were identified as endemic for the disease. High content of lanthanides in the soil in these regions generated a new geochemical hypothesis on the generation of this disease33,56. The high content of cerium in the diet which is fibrogenic for the heart, and the process is exacerbated because of the magnesium deficiency occurring in children because of malnutrition and diarrhoeal disorders57. The high level of cerium and relative magnesium deficiency noted in the cardiac tissues, could be shared by other organs of the body as well and so why the heart alone is affected remains unexplained58. By and large, poverty and eosinophilia are risk factors for developing the disease, and newer modes of evaluations could decipher the entire sequence of evolution59,60.
Changing natural history of endomyocardial fibrosis
Gupta and colleagues40 defined the natural history of the disease in Kerala in the late 1980s. Follow up of the initial 200 patients showed a 10 year survival of only 37 per cent40. Ascites, atrial fibrillation and New York Heart Association (NYHA) class IV were the poor prognostic indicators. Eighty nine patients, who underwent endocardiectomy with mitral valve replacement had an actuarial survival of 55 per cent during the same period1. Significant decline in the number of new cases happened in the hospital admissions in Kerala in the subsequent decades26. The new cases detected were incidental when evaluated for other cardiac disorders like coronary artery disease, and were a decade older. Natural history in them was more favourable with less than 10 per cent mortality on seven years follow up. The average number of cases seen declined by half in the last decade, compared to the previous decade. The mean age of the patients seen is now 33 yr compared to 25 yr in the previous decade, suggesting that people who were asymptomatic in the previous era are now being picked up on evaluation20. There are no patients below 10 yr, whereas in the previous decade, 28 per cent were below the age of 15 yr. The patients are less symptomatic and older. The majority are incidentally diagnosed when evaluated for electrocardiographic or echocardiographic abnormalities.
Temporal correlates of this changing natural history are worth analyzing. The period noted in the natural history studies belong to the 30 year period of 1976 to 200726,40. During the same period, Kerala witnessed substantial economic, nutritional and health transitions61. Cassava and plantain are no longer the staple diet for the Keralites. The per capita calorie consumption increased from 1600 to 2100 Kcals. The nutritional deficiency disorders were replaced by those of overnutrition and currently, Kerala is the diabetic capital for India62. Thanks to the good female literacy, health status of Kerala is acclaimed as an example for good health at low cost63. A community survey shows that there is a substantial decline in worm load per child64. The pot bellied children with multiple worm infestation, are no longer seen in the coastal belt of Kerala. Filarial endemicity continues to be little less, with rigorous governmental programmes initiated for its control65. Eosinophilia in children is now uncommon. There is substantial decline in rheumatic fever and rheumatic heart disease in children of Kerala correlating with the improved health care services and quality of life66. The question which needs to be answered now is what really caused this decline; is it the change in living standards, or change in the dietary pattern or the reduction in childhood infections? By and large, endomyocardial fibrosis could be a reaction pattern of the endocardium to a variety of insults29. Presence of interstitial fibrosis, myohypertrophy, and calcification speaks of the role of cytokines in its genesis1,29. Predominant right ventricular involvement in children could indicate an insult when the right ventricle could be more susceptible. Right ventricle receives most of the umbilical venous return in utero and is more dominant. But no antenatal cases are reported till date and the youngest report is that of a 4 month old baby67. The inflammatory response occurring in the younger age group could manifest as calcification in later years. Whether this calcification has its similarity to vascular and valvar calcium occurring in older age group; if so, could it be the factor which holds the key for unraveling this mystery?68
The time tunnel
Irrespective of intense multi faceted research, endomyocardial fibrosis continues to be an enigmatic disorder5. The specific endocardial involvements, localization to certain geographical pockets, propensity to affect the poor and typical endocardial calcification are the peculiarities of this disease. The slow disappearance of the disease from Kerala with improving socio-economic and health status adds credence to the nutritional and inflammatory components postulated in its genesis26,59. Dominant right ventricular involvement, argues for toxic factors which are removed at the lung, or a factor which affects the heart when the right ventricle is dominant or predisposed, as during in utero life. Advanced evaluation like extending to genomics and proteomics is likely to throw light on the final common pathway which leads to endocardial damage and fibrosis60.
Addendum: Subsequent to the preparation of this manuscript, there has been a report from South West Nigeria, by Akinwusi PO and Odeyemi AO [Clin Med Insights Cardiol 2012; 6 : 163-8] on the changing pattern of Endomyocardial fibrosis where they have observed a substantial decline in the number of clinical cases during similar time period.
References
- 1.Sivasankaran S. Restrictive cardiomyopathy in India: the story of a vanishing mystery. Heart. 2009;95:9–14. doi: 10.1136/hrt.2008.148437. [DOI] [PubMed] [Google Scholar]
- 2.Mocumbi AO, Yacoub S, Yacoub MH. Neglected tropical cardiomyopathies: II.Endomyocardial fibrosis: myocardial disease. Heart. 2008;94:384–90. doi: 10.1136/hrt.2007.136101. [DOI] [PubMed] [Google Scholar]
- 3.Mocumbi AO, Ferreira MB, Sidi D, Yacoub MH. A population study of endomyocardial fibrosis in a rural area of Mozambique. N Engl J Med. 2008;359:43–9. doi: 10.1056/NEJMoa0708629. [DOI] [PubMed] [Google Scholar]
- 4.Hutt MS. Epidemiology aspects of endomyocardial fibrosis. Postgrad Med J. 1983;59:142–6. doi: 10.1136/pgmj.59.689.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bukhman G, Ziegler J, Parry E. Endomyocardial fibrosis: still a mystery after 60 years. PLoS Negl Trop Dis. 2008;2:e97. doi: 10.1371/journal.pntd.0000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ball JD, Williams AW, Davies JN. Endomyocardial fibrosis. Lancet. 1954;266:1049–54. doi: 10.1016/s0140-6736(54)91619-0. [DOI] [PubMed] [Google Scholar]
- 7.Proceedings of the Cardiac Society of Great Britain and Ireland. Br Heart J. 1946;8:233–8. [No authors listed] [PMC free article] [PubMed] [Google Scholar]
- 8.Williams AW, Ball JD, Davies JN. Endomyocardial fibrosis in Africa: its diagnosis, distribution and nature. Trans R Soc Trop Med Hyg. 1954;48:290–305. doi: 10.1016/0035-9203(54)90100-5. [DOI] [PubMed] [Google Scholar]
- 9.Connor DH, Somers K, Hutt MSR, Manion WC, D’Arbela PG. Endomyocardial fibrosis in Uganda (Davies’ disease). Part I: An epidemiologic, clinical, and pathologic study. Am Heart J. 1967;74:687–709. doi: 10.1016/0002-8703(67)90509-1. [DOI] [PubMed] [Google Scholar]
- 10.Connor DH, Somers K, Hutt MS, Manion WC, D’Arbela PG. Endomyocardial fibrosis in Uganda (Davies’ disease). II. An epidemiologic, clinical, and pathologic study. Am Heart J. 1968;75:107–24. doi: 10.1016/0002-8703(68)90122-1. [DOI] [PubMed] [Google Scholar]
- 11.Vijayaraghavan G, Cherian G, Krishnaswami S, Sukumar IP. Left ventricular endomyocardial fibrosis in India. Br Heart J. 1977;39:563–8. doi: 10.1136/hrt.39.5.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nair DV. Endomyocardial fibrosis in Kerala State. Indian Heart J. 1982;34:412–7. [PubMed] [Google Scholar]
- 13.Reddy CR, Parvathi G, Rao NR. Pathology of cardiomyopathy from South India. Br Heart J. 1970;32:226–31. doi: 10.1136/hrt.32.2.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cherian G, Vijayaraghavan G, Krishnaswami S, Sukumar IP, John S, Jairaj PS, et al. Endomyocardial fibrosis: report on the hemodynamic data in 29 patients and review of the results of surgery. Am Heart J. 1983;105:659–66. doi: 10.1016/0002-8703(83)90491-x. [DOI] [PubMed] [Google Scholar]
- 15.Balakrishnan KG, Sapru RP, Sasidharan K, Venkitachalam CG. A comparison of the clinical, haemodynamic and angiographic features in right ventricular endomyocardial fibrosis and Ebstein's anomaly of the tricuspid valve. Cardiology. 1982;69:265–75. doi: 10.1159/000173515. [DOI] [PubMed] [Google Scholar]
- 16.Weber KT. Fruits and soil: a case for the equatorial doctor. Cardiovasc Res. 1995;30:635. [PubMed] [Google Scholar]
- 17.Berensztein CS, Piñeiro D, Marcotegui M, Brunoldi R, Blanco MV, Lerman J. Usefulness of echocardiography and doppler echocardiography in endomyocardial fibrosis. J Am Soc Echocardiogr. 2000;13:385–92. doi: 10.1016/s0894-7317(00)70008-3. [DOI] [PubMed] [Google Scholar]
- 18.Vijayaraghavan G, Davies J, Sadanandan S, Spry CJ, Gibson DG, Goodwin JF. Echocardiographic features of tropical endomyocardial disease in South India. Br Heart J. 1983;50:450–9. doi: 10.1136/hrt.50.5.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Diaz RA, Aranguiz E, Pedemonte O. Complementary roles of transthoracic two-dimensional color Doppler imaging and myocardial contrast echocardiography in diagnosis of endomyocardial fibrosis. Echocardiography. 2009;26:589–92. doi: 10.1111/j.1540-8175.2008.00847.x. [DOI] [PubMed] [Google Scholar]
- 20.Tharakan J. Electrocardiogram in endomyocardial fibrosis. Indian J Pacing Electrophysiol. 2011;11:129–33. [PMC free article] [PubMed] [Google Scholar]
- 21.Williams AW, Somers K. The electrocardiogram in endomyocardial fibrosis. Br Heart J. 1960;22:311–5. doi: 10.1136/hrt.22.3.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Somers K, Williams AW. The phonocardiogram in endomyocardial fibrosis. Br Heart J. 1960;22:546–50. doi: 10.1136/hrt.22.4.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Somers K, Williams AW. Intracardiac calcification in endomyocardial fibrosis. Br Heart J. 1962;24:324–8. doi: 10.1136/hrt.24.3.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Davies JN. The ridge in endomyocardial fibrosis. Lancet. 1968;1:631–2. doi: 10.1016/s0140-6736(68)91250-6. [DOI] [PubMed] [Google Scholar]
- 25.Pyrgakis VN, Shapiro LM, Donaldson RM. Unusual presentation of endomyocardial fibrosis. Int J Cardiol. 1988;20:409–12. doi: 10.1016/0167-5273(88)90298-7. [DOI] [PubMed] [Google Scholar]
- 26.Tharakan J, Bohora S. Current perspective on endomyocardial fibrosis. Curr Sci. 2009;97:405–10. [Google Scholar]
- 27.Freers J, Mayanja-Kizza H, Rutakingirwa M, Gerwing E. Endomyocardial fibrosis: why is there striking ascites with little or no peripheral oedema? Lancet. 1996;347:197. doi: 10.1016/s0140-6736(96)90383-9. [DOI] [PubMed] [Google Scholar]
- 28.Lowenthal MN, Teeger S. Endomyocardial fibrosis with pericardial effusion and endocardial calcification. Isr Med Assoc J. 2000;2:249. [PubMed] [Google Scholar]
- 29.Kartha CC. Endomyocardial fibrosis, a case for the tropical doctor. Cardiovasc Res. 1995;30:636–43. [PubMed] [Google Scholar]
- 30.Chopra P, Narula J, Talwar KK, Kumar V, Bhatia ML. Histomorphologic characteristics of endomyocardial fibrosis: An endomyocardial biopsy study. Hum Pathol. 1990;21:613–6. doi: 10.1016/s0046-8177(96)90007-6. [DOI] [PubMed] [Google Scholar]
- 31.Narayan GS, Sivasubramonian S. Spontaneous Fontan physiology in burnt-out endomyocardial fibrosis. Circulation. 2012;125:e296–7. doi: 10.1161/CIRCULATIONAHA.111.052514. [DOI] [PubMed] [Google Scholar]
- 32.Olsen EG, Spry CJ. Relation between eosinophilia and endomyocardial disease. Prog Cardiovasc Dis. 1985;27:241–54. doi: 10.1016/0033-0620(85)90008-8. [DOI] [PubMed] [Google Scholar]
- 33.Kutty VR, Abraham S, Kartha CC. Geographical distribution of endomyocardial fibrosis in South Kerala. Int J Epidemiol. 1996;25:1202–7. doi: 10.1093/ije/25.6.1202. [DOI] [PubMed] [Google Scholar]
- 34.Sapru RP. Proceedings of a Workshop held under the auspices of the Indian Council of Medical Research in Trivandrum, Kerala, India. September 30 and October 1, 1981. 1983. Endomyocardial fibrosis in India. [Google Scholar]
- 35.Valiathan MS, Balakrishnan KG, Kartha CC. A profile of endomyocardial fibrosis. Indian J Pediatr. 1987;54:229–36. doi: 10.1007/BF02750815. [DOI] [PubMed] [Google Scholar]
- 36.Davies H. Endomyocardial fibrosis and the tuberous diet. Int J Cardiol. 1990;29:3–8. doi: 10.1016/0167-5273(90)90265-7. [DOI] [PubMed] [Google Scholar]
- 37.Davies J, Spry CJ, Vijayaraghavan G, De Souza JA. A comparison of the clinical and cardiological features of endomyocardial disease in temperate and tropical regions. Postgrad Med J. 1983;59:179–85. doi: 10.1136/pgmj.59.689.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vijayaraghavan G, Sadanandan S. Immunological phenomena in tropical endomyocardial fibrosis. Indian Heart J. 1984;36:87–9. [PubMed] [Google Scholar]
- 39.Valiathan MS, Somers K, Kartha CC, editors. Oxford: Oxford University Press; 19993. Endomyocardial Fibrosis. [Google Scholar]
- 40.Gupta PN, Valiathan MS, Balakrishnan KG, Kartha CC, Ghosh MK. Clinical course of endomyocardial fibrosis. Br Heart J. 1989;62:450–4. doi: 10.1136/hrt.62.6.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sandhyamani S. Vasculopathic and cardiomyopathic changes induced by low-protein high-carbohydrate tapioca based diet in bonnet monkey. Vasculopathic and cardiomyopathic changes in induced malnutrition. Am J Cardiovasc Pathol. 1992;4:41–50. [PubMed] [Google Scholar]
- 42.Freers J, Amandua J, Mugerwa R. Endomyocardial fibrosis and eosinophilia. Lancet. 1993;342:1233. [PubMed] [Google Scholar]
- 43.Brockington IF, Olsen EG. Loffler's endocarditis and Davies’ endomyocardial fibrosis. Am Heart J. 1973;85:308–22. [Google Scholar]
- 44.Roberts WC, Liegler DG, Carbone PP. Endomyocardial disease and eosinophilia: A clinical and pathologic spectrum. Am J Med. 1969;46:28–42. doi: 10.1016/0002-9343(69)90055-2. [DOI] [PubMed] [Google Scholar]
- 45.Bijlsma F. Endomyocardial fibrosis and rheumatic heart disease in Mozambique. Trans R Soc Trop Med Hyg. 1979;73:661–2. doi: 10.1016/0035-9203(79)90015-4. [DOI] [PubMed] [Google Scholar]
- 46.Mocumbi AO, Yacoub MH, Yokohama H, Ferreira MB. Right ventricular endomyocardial fibrosis. Cardiovasc Pathol. 2009;18:64–5. doi: 10.1016/j.carpath.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 47.Shaper AG. What's new in endomyocardial fibrosis? Lancet. 1993;342:255–6. doi: 10.1016/0140-6736(93)91813-2. [DOI] [PubMed] [Google Scholar]
- 48.Connor DH, Somers K, Nelson AM, D’Arbela PG, Lukande R. The cause of endomyocardial fibrosis in Uganda. Trop Doct. 2012. [accessed on September 9, 2012]. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22875807 . [DOI] [PubMed]
- 49.McKinney B. Studies on the experimental production of endomyocardial fibrosis and cardiomegaly of unknown origin by dietary means. Am Heart J. 1975;90:206–14. doi: 10.1016/0002-8703(75)90121-0. [DOI] [PubMed] [Google Scholar]
- 50.Sezi CL. Effects of cassava diet on Cercopithecus aethiops livers: a case for cassava as the cause of both tropical splenomegaly syndrome (TSS) and endomyocardial fibrosis (EMF) East Afr Med J. 1996;73(Suppl 5):S24–8. [PubMed] [Google Scholar]
- 51.Williams AW. Endomyocardial fibrosis. Lancet. 1954;267:1075. doi: 10.1016/s0140-6736(54)90630-3. [DOI] [PubMed] [Google Scholar]
- 52.Crawford MA. Endomyocardial fibrosis and carcinoidosis. A common denominator? Am Heart J. 1963;66:273–6. doi: 10.1016/0002-8703(63)90046-2. [DOI] [PubMed] [Google Scholar]
- 53.Shaper AG. Plantain diets, serotonin, and endomyocardial fibrosis. Am Heart J. 1967;73:432–4. doi: 10.1016/0002-8703(67)90440-1. [DOI] [PubMed] [Google Scholar]
- 54.Marijon E, Ou P. What do we know about endomyocardial fibrosis in children of Africa? Pediatr Cardiol. 2006;27:523–4. doi: 10.1007/s00246-006-1262-y. [DOI] [PubMed] [Google Scholar]
- 55.Sezi CL. Endomyocardial fibrosis and eosinophilia. Lancet. 1993;342:1233–4. [PubMed] [Google Scholar]
- 56.Smith B, Chenery SRN, Cook JM, Styles MT, Tiberindwa JV, Hampton C, et al. Geochemical and environmental factors controlling exposure to cerium and magnesium in Uganda. J Geochem Exploration. 1998;65:1–15. [Google Scholar]
- 57.Valiathan SM, Kartha CC. Endomyocardial fibrosis - the possible connexion with myocardial levels of magnesium and cerium. Int J Cardiol. 1990;28:1–5. doi: 10.1016/0167-5273(90)90002-m. [DOI] [PubMed] [Google Scholar]
- 58.Vasan RS, Prakash GS. To believe or not to believe. Int J Cardiol. 1991;31:119–21. doi: 10.1016/0167-5273(91)90281-s. [DOI] [PubMed] [Google Scholar]
- 59.Rutakingirwa M, Ziegler JL, Newton R, Freers J. Poverty and eosinophilia are risk factors for endomyocardial fibrosis (EMF) in Uganda. Trop Med Int Health. 1999;4:229–35. doi: 10.1046/j.1365-3156.1999.43376.x. [DOI] [PubMed] [Google Scholar]
- 60.Wayengera M. Searching for new clues about the molecular cause of endomyocardial fibrosis by way of in silico proteomics and analytical chemistry. PLoS One. 2009;4:e7420. doi: 10.1371/journal.pone.0007420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sivasankaran S. Broadening waist line of Keralites, the diet link. Chapter 30. In: Kartha CC, editor. Kerala fifty years and beyond. Trivandrum, India: Gautha Books; 2007. pp. 307–44. [Google Scholar]
- 62.Mohan V, Sandeep S, Deepa R, Shah B, Varghese C. Epidemiology of type 2 diabetes: Indian scenario. Indian J Med Res. 2007;125:217–30. [PubMed] [Google Scholar]
- 63.Nath I, Reddy KS, Dinshaw KA, Bhisey AN, Krishnaswami K, Bhan MK, et al. Country profile: India. Lancet. 1998;351:1265–75. doi: 10.1016/s0140-6736(98)03010-4. [DOI] [PubMed] [Google Scholar]
- 64.Ramankutty V, Soman CR, Vijayakumar K. Pattern of helminthic infestation in primary schoolchildren of Thiruvananthapuram district. Kerala Research Programme on Local Development. Centre for Development Studies, Thiruvanathapuram. [accessed on September 5, 2012]. pp. 1–16. Discussion paper No. 19: 2000. Available from: http://www.cds.ac.in/krpcds/publication/ramankutty.htm .
- 65.Raju K, Jambulingam P, Sabesan S, Vanamail P. Lymphatic filariasis in India: Epidemiology and control measures. J Postgrad Med. 2010;56:232. doi: 10.4103/0022-3859.68650. [DOI] [PubMed] [Google Scholar]
- 66.Ramakrishnan S, Kothari SS, Juneja R, Bhargava B, Saxena A, Bahl VK. Prevalence of rheumatic heart disease: has it declined in India? Natl Med J India. 2009;22:72–4. [PubMed] [Google Scholar]
- 67.Aiello VD, Jatene MB. Early presentation of endomyocardial fibrosis. Cardiol Young. 2011;21:474. doi: 10.1017/S1047951111000278. [DOI] [PubMed] [Google Scholar]
- 68.Karwowski W, Naumnik B, Szczepañski M, Myśliwiec M. The mechanism of vascular calcification - a systematic review? Med Sci Monit. 2012;18:RA1–11. doi: 10.12659/MSM.882181. [DOI] [PMC free article] [PubMed] [Google Scholar]


