ABSTRACT
Although enteric human pathogens are usually studied in the context of their animal hosts, a significant portion of their life cycle occurs on plants. Plant disease alters the phyllosphere, leading to enhanced growth of human pathogens; however, the impact of human pathogens on phytopathogen biology and plant health is largely unknown. To characterize the interaction between human pathogens and phytobacterial pathogens in the phyllosphere, we examined the interactions between Pectobacterium carotovorum subsp. carotovorum and Salmonella enterica or Escherichia coli O157:H7 with regard to bacterial populations, soft rot progression, and changes in local pH. The presence of P. carotovorum subsp. carotovorum enhanced the growth of both S. enterica and E. coli O157:H7 on leaves. However, in a microaerophilic environment, S. enterica reduced P. carotovorum subsp. carotovorum populations and soft rot progression by moderating local environmental pH. Reduced soft rot was not due to S. enterica proteolytic activity. Limitations on P. carotovorum subsp. carotovorum growth, disease progression, and pH elevation were not observed on leaves coinoculated with E. coli O157:H7 or when leaves were coinoculated with S. enterica in an aerobic environment. S. enterica also severely undermined the relationship between the phytobacterial population and disease progression of a P. carotovorum subsp. carotovorum budB mutant defective in the 2,3-butanediol pathway for acid neutralization. Our results show that S. enterica and E. coli O157:H7 interact differently with the enteric phytobacterial pathogen P. carotovorum subsp. carotovorum. S. enterica inhibition of soft rot progression may conceal a rapidly growing human pathogen population. Whereas soft rotted produce can alert consumers to the possibility of food-borne pathogens, healthy-looking produce may entice consumption of contaminated vegetables.
IMPORTANCE
Salmonella enterica and Escherichia coli O157:H7 may use plants to move between animal and human hosts. Their populations are higher on plants cocolonized with the common bacterial soft rot pathogen Pectobacterium carotovorum subsp. carotovorum, turning edible plants into a risk factor for human disease. We inoculated leaves with P. carotovorum subsp. carotovorum and S. enterica or E. coli O157:H7 to study the interactions between these bacteria. While P. carotovorum subsp. carotovorum enhanced the growth of both S. enterica and E. coli O157:H7, these human pathogens affected P. carotovorum subsp. carotovorum fundamentally differently. S. enterica reduced P. carotovorum subsp. carotovorum growth and acidified the environment, leading to less soft rot on leaves; E. coli O157:H7 had no such effects. As soft rot signals a food safety risk, the reduction of soft rot symptoms in the presence of S. enterica may lead consumers to eat healthy-looking but S. enterica-contaminated produce.
Introduction
The gastrointestinal tract of animals has traditionally been considered the native ecological niche for enteric human pathogens such as Salmonella enterica and Escherichia coli O157:H7. However, accumulating evidence shows that both S. enterica and Shiga toxin-producing E. coli colonize and persist on plants in the environment, an important vehicle to humans (1). Over 1 million cases of food-borne illness linked to the consumption of contaminated fruits and vegetables are estimated to occur annually in the United States (2). Large, produce-linked outbreaks of S. enterica and Shiga toxin-producing E. coli highlight the inadequacy of our understanding of the biology of these food-borne human pathogens outside their animal hosts (3).
The plant phyllosphere is believed to be inhospitable to many microorganisms, such as enteric human pathogens, due to acute fluctuations in environmental conditions, such as temperature, UV radiation, and water and nutrient availability (4, 5). Interactions with plant microflora may contribute to the success of human pathogens on plants by facilitating nutrient and water acquisition and access to the more sheltered plant interior, e.g., through plant cell wall degradation (6), providing protection against environmental stresses in multispecies biofilms (7) or downregulation of plant defenses (8). Previous studies have shown that the actions of phytopathogens improve the survival of human pathogens on plants (4, 5, 9–11). However, information on the impact of enteric human pathogens on the biology of phytobacterial pathogens in planta is extremely limited (4, 5, 11). Understanding both the human pathogen and phytobacterial components of this interaction is essential to gain a broad understanding of the ecology of human pathogens on plants.
Pectobacterium carotovorum subsp. carotovorum is a ubiquitous plant pathogen that causes bacterial soft rot on nearly every type of fruit and vegetable produced in tropical and temperate regions (12–14). Infection by this bacterial phytopathogen causes water-soaked lesions and rapid maceration of plant tissues. P. carotovorum subsp. carotovorum pathogenesis is mediated by a large repertoire of cell wall-degrading enzymes (CWDEs) secreted by the type II secretion system. The many pectinases, cellulases, and xylanases secreted allow P. carotovorum subsp. carotovorum to acquire nutrients from a wide range of hosts and are major virulence factors—gene products that increase the amount of disease—of this pathogen (15–17). Mutation of the type II secretion system inhibits plant tissue maceration and the consequent expansion of the infection by P. carotovorum subsp. carotovorum (18), illustrating the importance of CWDEs in plant pathogenesis (15, 17). In addition to CWDEs, other P. carotovorum subsp. carotovorum virulence factors include type III effectors (19), a necrosis-inducing protein (20, 21), and the catabolic α-acetolactate synthase BudB (22). BudB catalyzes the first step in the butanediol (bud) pathway for environmental alkalinization. Alkaline pH is required for efficient plant tissue degradation by pectate lyases, the main CWDE for P. carotovorum subsp. carotovorum soft rot progression (15–17, 22, 23). Failure by a P. carotovorum subsp. carotovorum budB mutant to elevate plant tissue pH reduced soft rot by ~70% on potato tubers (22).
Association of Salmonella and bacterial soft rot caused by P. carotovorum subsp. carotovorum was discovered in market surveys (9). Incidence of Salmonella ranges from 1 to 10% on “healthy,” unblemished produce but rises to 18 to 20% in the presence of bacterial soft rot. Thus, edible plants are a highly relevant but largely uninvestigated ecological niche for human pathogens. To better characterize the interaction between enteric human pathogens and soft rot bacteria in this environment, we examined the interactions between S. enterica or E. coli O157:H7 and P. carotovorum subsp. carotovorum in postharvest lettuce (Lactuca sativa). Bacterial populations, soft rot progression, and changes in local pH were observed for up to 96 h postinoculation (hpi). Results from this study highlight distinct differences between S. enterica and E. coli O157:H7 in their interactions with phytopathogenic bacteria. This study is also the first to examine the impact of enteric human pathogens on plant pathogen growth, phytobacterial pathogen-plant interactions, and plant disease progression.
RESULTS
Enhanced growth of S. enterica by P. carotovorum subsp. carotovorum is concurrent with reduced populations of the phytopathogen in a microaerophilic environment.
Based on previous studies of the growth of human pathogens in the presence of plant pathogens on plants, we hypothesized that (i) S. enterica would attain higher populations in the presence of P. carotovorum subsp. carotovorum and (ii) S. enterica growth would reduce P. carotovorum subsp. carotovorum proliferation. We conducted our experiments in modified atmosphere packaging (MAP) to reflect conditions found in packaged, bagged lettuce. MAP is characterized by low O2 and high CO2 concentrations that are maintained by selectively permeable polymeric films used for packaging (24). Across five experiments, differences in the mean populations of single- and coinoculated S. enterica and P. carotovorum subsp. carotovorum on lettuce leaves in a microaerophilic environment were seen as early as 24 hpi (Tukey’s honestly significant difference [HSD] test, n = 135, P < 0.0001) through 96 hpi (Fig. 1A). At 96 hpi, the mean populations of S. enterica alone on leaves ranged from 7.58 to 8.45 log10 CFU g−1 but reached 8.99 to 9.82 log10 CFU g−1 in the presence of wild-type (WT) P. carotovorum subsp. carotovorum. E. coli O157:H7 also attained larger populations when it was coinoculated with WT P. carotovorum subsp. carotovorum (Fig. 1B) (Tukey’s HSD test, n = 53); on average, across all experiments, E. coli O157:H7 populations were 1 log10 CFU g−1 higher at 96 hpi. Analysis of variance (ANOVA) showed a statistically significant interaction between experiment and treatment for both S. enterica populations (degrees of freedom [df] = 8, F = 3.41, P = 1.48e−03) and E. coli populations (df = 4, F = 5.23, P = 1.01e−03). Growth promotion of enteric human pathogens on plants appears to be a common outcome of their interaction with the soft rot pathogen P. carotovorum subsp. carotovorum.
FIG 1 .
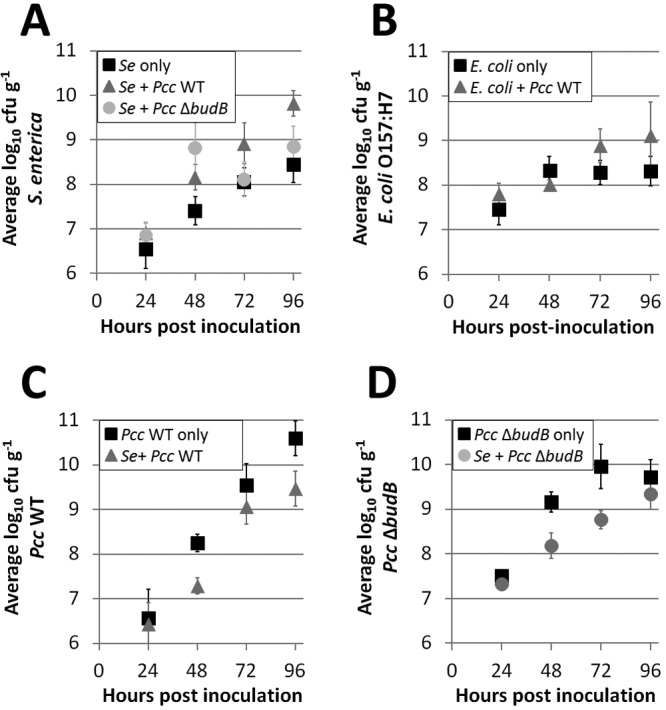
Population dynamics on lettuce in a microaerophilic environment. Growth of S. enterica (Se) (A) and E. coli O157:H7 (B) was enhanced in the presence of P. carotovorum subsp. carotovorum (Pcc). In contrast, WT P. carotovorum subsp. carotovorum (C) and budB mutant (D) growth was reduced when lettuce leaves were coinoculated with S. enterica. Data shown are the means of nine samples per treatment from a representative experiment. Vertical bars indicate the standard deviations.
We also examined the growth of S. enterica coinoculated with a P. carotovorum subsp. carotovorum budB mutant, which is reduced in virulence. Across five experiments, S. enterica populations ranged from 7.69 to 9.16 log10 CFU g−1 at 96 hpi in the presence of the P. carotovorum subsp. carotovorum budB mutant, an increase of 0.44 to 1.41 log10 CFU g−1 over S. enterica populations on leaves inoculated with S. enterica alone. While significant, this increase was less than the increase seen with S. enterica coinoculated with WT P. carotovorum subsp. carotovorum (P < 0.0001) at 96 hpi. The increases in population means observed at 96 hpi for S. enterica in the presence of either WT P. carotovorum subsp. carotovorum, the P. carotovorum subsp. carotovorum budB mutant, or S. enterica alone were statistically significant (Tukey’s HSD test, n = 134, P < 0.0001) (Fig. 1A).
In contrast, P. carotovorum subsp. carotovorum populations were reduced in the presence of S. enterica from 48 to 96 hpi (Fig. 1C and D). The populations of both WT P. carotovorum subsp. carotovorum and the P. carotovorum subsp. carotovorum budB mutant on leaves exceeded 9.7 log10 CFU g−1 at 96 hpi in all five experiments when these bacteria were inoculated alone, and these populations were not statistically different from each other (Tukey’s HSD test, n = 180, P > 0.1). Across all five experiments, when coinoculated with S. enterica, the average populations of WT P. carotovorum subsp. carotovorum and the P. carotovorum subsp. carotovorum budB mutant were both reduced by roughly 1 log10 CFU g−1 at 96 hpi (P < 0.0001) (Fig. 1C and D). The means of the two P. carotovorum subsp. carotovorum strains were not statistically different from each other (Tukey’s HSD test, n = 180, P > 0.05), indicating that the budB mutation has no effect on P. carotovorum subsp. carotovorum bacterial populations by 96 hpi, regardless of the presence of S. enterica. ANOVA showed a statistically significant interaction between experiment and treatment (df = 12, F = 4.24, P = 8.69e−06).
Soft rot disease progression is delayed in the presence of S. enterica in a microaerophilic environment.
To investigate whether the P. carotovorum subsp. carotovorum population reduction on leaves coinoculated with S. enterica resulted in a decline in soft rot disease, we measured lesion length. Lesions consisting of watery, macerated plant tissue were observed on leaves inoculated with WT P. carotovorum subsp. carotovorum or the P. carotovorum subsp. carotovorum budB mutant but not S. enterica alone. The average length of lesions produced by WT P. carotovorum subsp. carotovorum was longer than the average lesion lengths in all other treatments at 24 hpi, 48 hpi, and 72 hpi (Fig. 2A to C). The lengths of lesions were reduced on leaves coinoculated with either P. carotovorum subsp. carotovorum strain and S. enterica and on leaves treated with the P. carotovorum subsp. carotovorum budB mutant alone. Bacterial soft rot was observed as early as 24 hpi on WT P. carotovorum subsp. carotovorum-treated leaves, whereas, across five experiments, lesions did not develop until 48 hpi on leaves inoculated with the P. carotovorum subsp. carotovorum budB mutant or coinoculated with S. enterica and either P. carotovorum subsp. carotovorum strain. Lesion lengths of ≤3 mm were considered equal and indistinguishable from the inoculation wound. At 72 hpi, the mean length of lesions observed on leaves inoculated with WT P. carotovorum subsp. carotovorum was substantially greater than that of leaves coinoculated with WT P. carotovorum subsp. carotovorum and S. enterica, which was significantly greater than the average lesion length on leaves inoculated with the P. carotovorum subsp. carotovorum budB mutant or coinoculated with the P. carotovorum subsp. carotovorum budB mutant and S. enterica (Tukey’s HSD test, n = 240, P < 0.001) (Fig. 2C). ANOVA showed a statistically significant interaction between experiment and treatment at 24 h (df = 20, F = 1.65, P = 0.044) and 48 h (df = 12, F = 9.99, P = 1.90e−14) but not at 72 h (df = 12, F = 1.45, P = 0.1480). Lesion lengths are not reported at 96 hpi because entire leaves were typically consumed by soft rot and symptoms caused by P. carotovorum subsp. carotovorum could no longer be distinguished from those resulting from possible secondary infections.
FIG 2 .
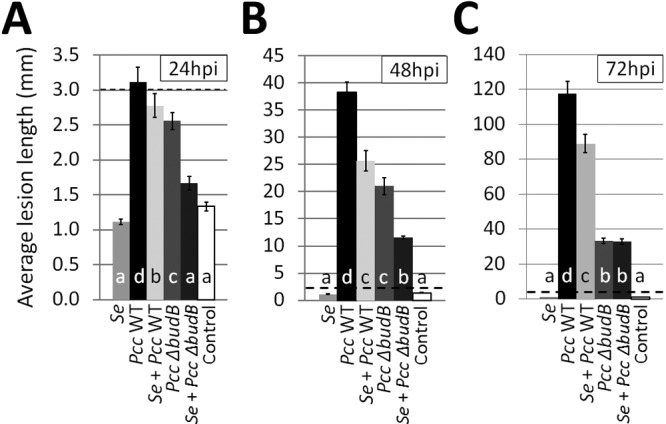
P. carotovorum subsp. carotovorum virulence was attenuated in the budB mutant and in the presence of the human pathogen S. enterica. Soft rot lesion length was measured at 24 hpi (A), 48 hpi (B), and 72 hpi (C). Note the difference in scale between graphs. All lesion lengths below 3 mm (black dotted line) were considered to be equal due to variability in puncture wound size. Data shown are the means of nine samples per treatment from a representative experiment. Vertical bars indicate the standard errors.
S. enterica suspends the relationship between the P. carotovorum subsp. carotovorum population and lesion size.
As the population of WT P. carotovorum subsp. carotovorum on leaves increased, the length of soft rot lesions also increased (Fig. 1C and 2). Surprisingly, a plot of the WT P. carotovorum subsp. carotovorum population against the length of soft rot lesions on leaves showed only a moderate correlation between the two variables (Fig. 3A). This relationship, described by the slope of the line (β), was weakened by the presence of S. enterica. When coinoculated with S. enterica, WT P. carotovorum subsp. carotovorum reached lower population densities and more soft rot bacteria were needed to produce the same size of lesions as in the WT P. carotovorum subsp. carotovorum-only treatment (Fig. 3B). S. enterica also moderated WT P. carotovorum subsp. carotovorum disease progression such that the slope more closely resembled that of disease progression in leaves singly inoculated with the P. carotovorum subsp. carotovorum budB mutant. This indicated that S. enterica interfered with the ability of P. carotovorum subsp. carotovorum to rot plant tissues.
FIG 3 .
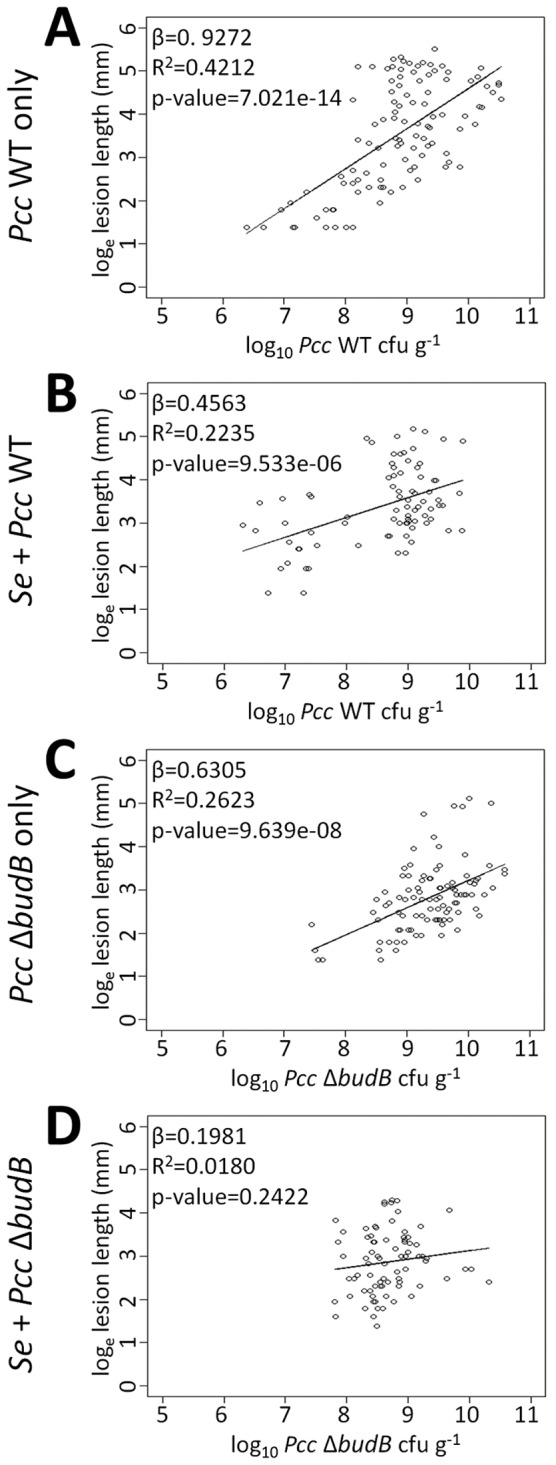
S. enterica disrupts the correlation between P. carotovorum subsp. carotovorum population and lesion size. Populations of WT P. carotovorum subsp. carotovorum or the P. carotovorum subsp. carotovorum budB mutant were plotted against the observed soft rot lesion length (loge transformed) from 24 hpi to 72 hpi to show the dependency of lesion length on bacterial density. Linear regression lines are shown, with slope (β) and R2 values indicated. A moderate relationship between the WT P. carotovorum subsp. carotovorum population and lesion size exists (A); however, the slope of the line changed in the presence of S. enterica (B) or deletion of budB (C). No relationship between the P. carotovorum subsp. carotovorum budB mutant population and lesion size can be discerned in the presence of S. enterica (D). The P values indicate the probability that the P. carotovorum subsp. carotovorum population had no effect on lesion length, i.e., the H0 of β = 0 is true.
budB is an important fitness factor for P. carotovorum subsp. carotovorum in the presence of S. enterica at middisease.
Deletion of budB did not reduce P. carotovorum subsp. carotovorum replication on leaves when the bacteria were inoculated alone. However, in the presence of S. enterica, the fitness of the P. carotovorum subsp. carotovorum budB mutant was significantly reduced middisease, as indicated by retarded replication and subsequent soft rot lesion development within 72 hpi. Replication of the P. carotovorum subsp. carotovorum budB mutant in the first 72 h was severely restricted beyond 2 × 109 CFU g−1 compared to WT P. carotovorum subsp. carotovorum (Fig. 3D). Deletion of the budB gene also weakened the relationship, described by the slope of the line (β), between the population of P. carotovorum subsp. carotovorum and observed lesion length, meaning that a higher P. carotovorum subsp. carotovorum budB mutant population was necessary to produce the same size of lesions as WT P. carotovorum subsp. carotovorum (Fig. 3A and C). The P. carotovorum subsp. carotovorum budB mutant required populations of at least log10 7.5 CFU g−1 for disease development. Regardless of population, the P. carotovorum subsp. carotovorum budB mutant rarely produced lesions larger than 55 mm (4 loge in Fig. 3C). In leaves coinoculated with the P. carotovorum subsp. carotovorum budB mutant and S. enterica, there was little increase in lesion size as the population of the P. carotovorum subsp. carotovorum budB mutant enlarged and its population was no longer a predictor of lesion size (P > 0.1 for the null hypothesis [H0] of β = 0 in Fig. 3D). Therefore, the budB mutant was compromised in plant colonization by S. enterica.
Diminished soft rot symptoms in the presence of S. enterica are accompanied by a reduction in pH.
In a microaerophilic environment, such as MAP, S. enterica uses mixed acid fermentation for energy production, generating organic acids such as acetate, formate, and succinate (25). P. carotovorum subsp. carotovorum also utilizes acid fermentation in oxygen-limited conditions. Having observed diminished soft rot by the P. carotovorum subsp. carotovorum budB mutant, which cannot neutralize fermentation acids to elevate local pH, we speculated that S. enterica moderates P. carotovorum subsp. carotovorum soft rot disease progression by acidifying the local pH in leaves, thereby reducing the efficiency of plant tissue maceration by pectate lyases. To test this hypothesis, we determined the pH of leaf tissue surrounding the site of bacterial inoculation. The pH of leaves inoculated with S. enterica was significantly lower than the pH of water-inoculated (control) leaves at 96 hpi (P = 0.04). In contrast, the pH of leaves inoculated with WT P. carotovorum subsp. carotovorum steadily increased over 96 h (Fig. 4A to D), concurrent with the observed increase in lesion length (Fig. 2A to C). At 48 hpi, 72 hpi, and 96 hpi, the mean pH of WT P. carotovorum subsp. carotovorum-inoculated leaves was significantly greater than the pH means of all other treatments. The pH of leaves coinoculated with WT P. carotovorum subsp. carotovorum and S. enterica was similar to that of the S. enterica-inoculated leaves from 24 to 72 hpi (Fig. 4A to C) and significantly lower than that of the WT P. carotovorum subsp. carotovorum-inoculated leaves at all sampling intervals. The pH of the P. carotovorum subsp. carotovorum budB mutant-treated leaves increased during the first 48 hpi but then declined over the next 48 h to resemble that of the water-inoculated control. Across all five experiments, the pH of leaves coinoculated with the P. carotovorum subsp. carotovorum budB mutant and S. enterica was similar to that of the water control at all sampling intervals (Tukey’s HSD test, n = 240, P > 0.05). ANOVA showed a statistically significant interaction between experiment and treatment at 24 h (df = 20, F = 2.32, P = 1.65e−03), 48 h (df = 20, F = 5.63, P = 1.60e−11), 72 h (df = 20, F = 3.66, P = 1.01e−06), and 96 h (df = 20, F = 3.11, P = 2.28e−05). Taken together, these data suggest that S. enterica interferes with the ability of P. carotovorum subsp. carotovorum to alkalinize plant tissues and thus reduced the activity of pectate lyases, in part by directly acidifying the environment.
FIG 4 .
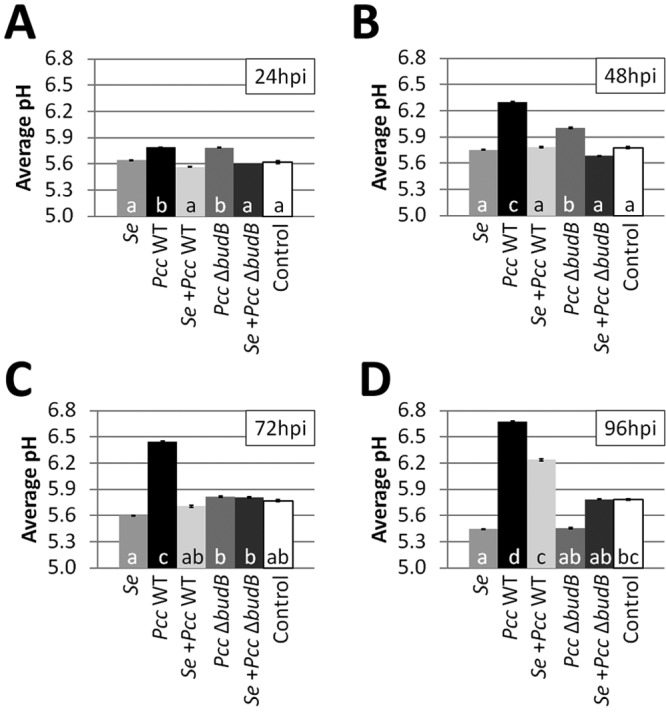
Changes in leaf pH in microaerophilic conditions. The pH of detached leaves inoculated with WT P. carotovorum subsp. carotovorum or the P. carotovorum subsp. carotovorum budB mutant and/or S. enterica was determined spectrophotometrically every 24 h for 96 h (A to D). The pH of leaf tissue inoculated with WT P. carotovorum subsp. carotovorum increases toward neutral over time, but this increase was diminished in the presence of S. enterica. Though there was a pH increase in the P. carotovorum subsp. carotovorum budB mutant-inoculated leaves at 24 hpi and 48 hpi (A and B, respectively), the leaf tissue acidified and resembled the water-inoculated control at 72 hpi and 96 hpi (C and D, respectively). At 96 hpi, the pH of leaf tissue inoculated with S. enterica was lower than that of the water-inoculated control, presumably due to accumulation of acids produced in anaerobic conditions (D). Data shown are the averages of five experiments. Vertical bars indicate the standard errors.
S. enterica has no effect on the P. carotovorum subsp. carotovorum population and soft rot disease progression in aerobic conditions.
To test our hypothesis that S. enterica interferes with soft rot progression by acidifying the phyllosphere, thereby attenuating alkalinization by P. carotovorum subsp. carotovorum, we repeated our detached-leaf experiments in aerobic conditions. When oxygen is available, S. enterica depends on aerobic pathways of energy production, such as through oxidative phosphorylation, and thus does not produce measurable amounts of fermentation acids. In aerobic conditions, S. enterica had no effect on the WT P. carotovorum subsp. carotovorum population, disease progression, or pH (see Fig. S1 in the supplemental material) (24-hpi and 48-hpi data from all three experiments were pooled; Tukey’s HSD test, npopulation = 212 or nlesion and pH = 216, P > 0.05). These data support our hypothesis that S. enterica restriction of pH elevation plays a significant role in reducing P. carotovorum subsp. carotovorum soft rot in a microaerophilic environment. The availability of oxygen had no impact on the growth enhancement of S. enterica by WT P. carotovorum subsp. carotovorum. S. enterica populations were roughly 1 log10 higher in the presence of WT P. carotovorum subsp. carotovorum, similar to the enhanced growth of S. enterica in the presence of P. carotovorum subsp. carotovorum that was observed in a microaerophilic environment (Fig. 1A; Fig. S1A).
Reduced soft rot is not due to S. enterica proteolytic inactivation of P. carotovorum subsp. carotovorum CWDEs.
Because bacterial soft rot progression is dependent on the activity of P. carotovorum subsp. carotovorum pectolytic enzymes, we investigated whether the reduction in soft rot in the presence of S. enterica was due to proteolytic inactivation of these enzymes by S. enterica. Plant cell wall degradation by P. carotovorum subsp. carotovorum CWDEs leads to lysis of host cells. Leaf lysate is nutritionally analogous to ruptured plant cells that are lysed and have released their contents (26). S. enterica had no detectable proteolytic activity in leaf lysate in a microaerophilic environment (Fig. 5A and B). In aerobic conditions, S. enterica produced small, faint halos, indicating minor proteolytic activity (Fig. 5C and D), but this did not appear to interfere with soft rot progression (see Fig. S1B in the supplemental material). Under both microaerophilic and aerobic conditions, P. carotovorum subsp. carotovorum exhibited proteolytic activity (Fig. 5), consistent with reported protease secretion through the type I and II secretion pathways (16, 23). S. enterica reduced P. carotovorum subsp. carotovorum proteolytic activity in aerobic conditions. We posit that the reduction in proteolytic activity was due to a reduction in the P. carotovorum subsp. carotovorum population grown in leaf lysate (Table S1) which does not occur in the phyllosphere. Due to the reduction in the P. carotovorum subsp. carotovorum population when cocultured with S. enterica in leaf lysate, it would be difficult to distinguish between reduced proteolytic activity due to a reduced P. carotovorum subsp. carotovorum population and enzymatic inhibition by S. enterica following a 1:1 inoculation, as shown in Fig. 5B and D. We inoculated leaf lysate cocultures with 10× more P. carotovorum subsp. carotovorum so that at 24 hpi the P. carotovorum subsp. carotovorum population in the presence of S. enterica would be similar to the P. carotovorum subsp. carotovorum population in the absence of S. enterica (Fig. 5A and C). No change in proteolytic activity was observed for the coculture of P. carotovorum subsp. carotovorum and S. enterica grown in leaf lysate, indicating that S. enterica does not produce an extracellular protease in the presence of P. carotovorum subsp. carotovorum and that S. enterica does not inhibit P. carotovorum subsp. carotovorum proteolytic activity. Taken together, our data indicate that the suppression of soft rot by S. enterica was not due to proteolytic inactivation of P. carotovorum subsp. carotovorum CWDEs.
FIG 5 .
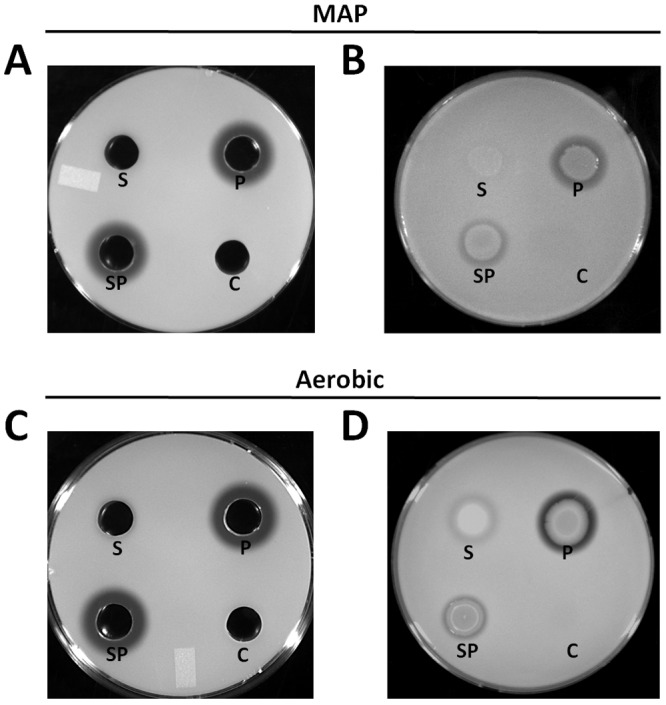
S. enterica exhibited no proteolytic activity in microaerophilic conditions (A and B) and minor proteolytic activity in aerobic conditions (C and D). Proteolytic activity of S. enterica and WT P. carotovorum subsp. carotovorum cultured in leaf lysates under microaerophilic (A and B) or aerobic (C and D) conditions. Filtered supernatants from 24-h lettuce lysate cultures of S. enterica and WT P. carotovorum subsp. carotovorum or a coculture of these bacteria were applied to wells in milk agar-lettuce lysate plates to assay cell-free proteolytic activity (A and C). Twenty-four-hour leaf lysate cultures were spotted onto plates to evaluate both cell-associated and cell-free extracellular proteolytic activity (B and D). Proteolytic activity is evident as a zone of clearing around the well or bacterial colony observed after 48 h of incubation at 28°C. Abbreviations: S, S. enterica; P, WT P. carotovorum subsp. carotovorum; SP, coculture of S. enterica and WT P. carotovorum subsp. carotovorum; C, control. Plates are representative replicates from one experiment. There were three replicates per treatment, and the experiment was repeated three times.
E. coli has no effect on the P. carotovorum subsp. carotovorum population, soft rot disease progression, or pH in a microaerophilic environment.
Having observed that S. enterica affects the P. carotovorum subsp. carotovorum population, disease progression, and local pH modification on leaves in a microaerophilic environment and that P. carotovorum subsp. carotovorum enhances the growth of S. enterica, we asked whether these effects were common outcomes of the interaction between P. carotovorum subsp. carotovorum and enteric human pathogens. In a microaerophilic environment, coinoculation of leaves with WT P. carotovorum subsp. carotovorum and E. coli O157:H7 enhanced the growth of E. coli but had no effect on the WT P. carotovorum subsp. carotovorum population, disease progression, or pH at 24 hpi, 48 hpi, or 72 hpi (see Fig. S2 in the supplemental material) (data pooled from all three experiments; Tukey’s HSD test, npopulation = 53 or nlesion and pH = 90, P > 0.05).
DISCUSSION
In planta growth promotion of enteric human pathogens by the actions of phytopathogenic bacteria in aerobic conditions has been reported (4, 5, 9, 10, 27). In the present study, the S. enterica and E. coli O157:H7 populations were enhanced 2- to 20-fold and 3- to 15-fold, respectively, at 48 hpi in the presence of WT P. carotovorum subsp. carotovorum in aerobic conditions, consistent with previous reports of growth with other bacterial soft rot pathogens in aerobic conditions on a variety of plants. S. enterica populations in aerobic conditions increased 10-fold on potato, carrot, and pepper disks coinoculated with Dickeya dadantii (formerly Erwinia chrysanthemi) (9), and E. coli O157:H7 populations increased 3.3- to 6.2-fold and 10-fold on lettuce leaves coinoculated with D. dadantii (5, 10). The focus in this study was the interaction between human and plant pathogens in a microaerophilic environment in which we found that the average population of S. enterica was 6- to 37-fold higher and the average population of E. coli O157:H7 was 4- to 85-fold higher at 96 hpi in the presence of WT P. carotovorum subsp. carotovorum.
Information on the impact of common food-borne pathogens, such as S. enterica and Shiga toxin-producing E. coli, on plant pathogen growth and virulence is limited to brief mentions in three reports by Aruscavage et al. (4), Noel et al. (11), and Yamazaki et al. (5). Our study is the first to focus on the effect human pathogens have on plant pathogens. We showed that S. enterica limits P. carotovorum subsp. carotovorum population growth and bacterial soft rot development on leaves in a microaerophilic environment and that this was correlated with diminished environmental pH elevation by P. carotovorum subsp. carotovorum. The reduction in WT P. carotovorum subsp. carotovorum populations corresponded to a reduction in the length of soft rot lesions (Fig. 2). If the reduction in the WT P. carotovorum subsp. carotovorum population and soft rot disease progression was solely the result of competition, similarly sized lesions would have been expected to contain similar populations of WT P. carotovorum subsp. carotovorum regardless of the presence or absence of S. enterica. The reduction in disease progression observed in the presence of S. enterica would have resulted only from limitation of WT P. carotovorum subsp. carotovorum growth, and there would be no effect on the relationship between the WT P. carotovorum subsp. carotovorum population and soft rot lesion length. Instead, our data revealed that higher populations of WT P. carotovorum subsp. carotovorum were necessary to produce the same sizes of lesions in the presence of S. enterica (Fig. 3A and B), indicating that S. enterica likely utilizes strategies other than competition to reduce bacterial soft rot.
pH elevation is especially important in promoting the multiplication of enteric human pathogens in soft rot lesions. Compared to coinoculation with WT P. carotovorum subsp. carotovorum, a weaker (2- to 18-fold) population increase for S. enterica was observed at 96 hpi when S. enterica was coinoculated with a P. carotovorum subsp. carotovorum budB mutant defective in environmental pH neutralization, indicating that pH elevation is important for S. enterica growth in this niche.
The ability to alkalinize the pH of the plant host, e.g., through the bud pathway, is integral to soft rot progression. Pectate lyases, the major enzymes responsible for plant tissue maceration, have pH optima between 8.0 and 8.5 (15, 17, 23). The plant apoplastic pH is typically 5.5 to 6.0, at which the activities of pectate lyases are low (17, 23). The activity of the pectate lyase PelC is 100-fold less at pH 6 than at the pH optimum of 8.5 in D. dadantii (28). Accordingly, a P. carotovorum subsp. carotovorum budB mutant was observed to be reduced in virulence, i.e., it produced less macerated tissue, in leaves (this study) and potato tubers (22). Mutation of budB in the related phytopathogen D. dadantii also reduced its virulence on potato and chicory (29)—confirming the role of budB as a soft rot virulence factor. In this study, the presence of S. enterica exacerbated the virulence defect of the P. carotovorum subsp. carotovorum budB mutant. Without the bud pathway and in the presence of S. enterica, soft rot lesion expansion was restricted and more cells were necessary to form lesions. Additionally, when coinoculated with S. enterica, replication of the P. carotovorum subsp. carotovorum budB mutant slowed during middisease development, indicating that budB is also a fitness factor for P. carotovorum subsp. carotovorum.
Bacterial soft rot progression is dependent on the activity of P. carotovorum subsp. carotovorum pectinolytic enzymes whose efficiencies peak at alkaline pH. When butanediol production is eliminated in P. carotovorum subsp. carotovorum or D. dadantii bud pathway mutants, infected plant tissues are more acidic and disease is reduced compared with those inoculated with the WT (22, 29). The importance of pH in the progression of soft rot caused by P. carotovorum subsp. carotovorum was also reported by Ni et al. (30), who observed a 50 to 70% reduction in calla lily tuber rot at pH 6.3 compared with that at pH 7.0 and 7.3. We observed a reduction in soft rot lesion size on leaves inoculated with the P. carotovorum subsp. carotovorum budB mutant or coinoculated with WT P. carotovorum subsp. carotovorum or the P. carotovorum subsp. carotovorum budB mutant and S. enterica, a decrease which was accompanied by a reduction in the local pH relative to samples inoculated with WT P. carotovorum subsp. carotovorum only. The pH of leaves inoculated with S. enterica only was lower than that of the negative control. In contrast, under aerobic conditions in which S. enterica does not produce a measurable amount of fermentation acids, the pH of leaves inoculated with S. enterica was similar to that of the negative control. Furthermore, coinoculation of leaves with S. enterica had no effect on the pH or disease progression by WT P. carotovorum subsp. carotovorum under aerobic conditions. We interpret these results to mean that S. enterica acidifies the phyllosphere in microaerophilic conditions due to production of organic acids during fermentation, thereby reducing the efficiency of plant tissue degradation by pectate lyases. Since soft rot is mediated by CWDEs, we verified that the antagonistic effect of S. enterica on P. carotovorum subsp. carotovorum is not due to proteolytic inactivation of these enzymes. No S. enterica proteolytic activity was observed in the microaerophilic environment, and the small amounts of proteolytic activity in aerobic conditions were not accompanied by an effect on P. carotovorum subsp. carotovorum populations or disease progression. We conclude that the mechanism by which S. enterica suppresses P. carotovorum subsp. carotovorum bacterial soft rot is through attenuation of P. carotovorum subsp. carotovorum pH environmental alkalinization.
In contrast to our results with S. enterica, coinoculation with E. coli O157:H7 had no effect on the population or soft rot disease progression in a microaerophilic environment. Previous studies by Aruscavage et al. (4) and Yamazaki et al. (5) also showed that coinoculation of leaves with E. coli O157:H7 had no effect on the populations of Xanthomonas campestris pv. vitians and D. dadantii, respectively. We found that E. coli O157:H7 by itself failed to acidify leaf tissue, unlike S. enterica. We conclude that the null effect of E. coli O157:H7 on P. carotovorum subsp. carotovorum is due to the inability of E. coli O157:H7 to acidify plant tissue. Without environmental acidification, P. carotovorum subsp. carotovorum pectate lyase efficiency is not affected and, thus, soft rot development proceeds similarly to that with P. carotovorum subsp. carotovorum alone. Our observations also indicate that the E. coli O157:H7-P. carotovorum subsp. carotovorum interaction is fundamentally different from the S. enterica interaction. Although both E. coli and S. enterica use mixed acid fermentation in anaerobic conditions, the pH of tissue inoculated with E. coli O157:H7 was similar to that of the water control, whereas S. enterica acidified the leaves. If the substrates are available in anaerobic conditions, E. coli will use anaerobic respiration with nitrate or fumarate as an electron receptor instead of fermentation (31–33). Lettuce leaves have previously been shown to contain significant levels of nitrate (34). It is likely that the nutrients, e.g., nitrate, made available during leaf maceration by P. carotovorum subsp. carotovorum, lead to E. coli forgoing fermentation for the more energetically favorable anaerobic respiration, which will lead to lower acid production.
We strived to replicate natural conditions in our experimental design. Plant tissue damage resulting from our inoculation technique mimics the mechanical damage associated with handling during and after harvest. Compared to previous studies, our low inocula of P. carotovorum subsp. carotovorum and S. enterica may better reflect the natural populations of these bacteria encountered on leaves in the field or postharvest. The microaerophilic conditions achieved in this study (<1% O2, >13% CO2) are similar to conditions found in MAP (0.5 to 3% O2, 10 to 15% CO2) for produce (35, 36). Processed fruits and vegetables are frequently placed in MAP to extend shelf life by reducing the rate of plant respiration and ethylene production (senescence) and the growth of microorganisms responsible for spoilage (24). However, MAP has no effect on replication or survival of enteric human pathogens on raw fruits and vegetables (37). Bagged lettuce, packaged in MAP, is the fastest growing segment of lettuce consumption (38). To our knowledge, this is the first study examining the interactions between food-borne enteric human pathogens and bacterial phytopathogens on fresh produce in MAP. By conducting our experiments at 24°C, we illustrate the bacterial interactions that may occur on poorly handled packaged produce.
Soft rot serves as an important cue deterring human consumption of fruits and vegetables mishandled during harvest, during processing, along the supply chain, or by the consumer. While produce may be contaminated by Salmonella in the absence of plant pathogens, the presence of phytopathogens doubled the incidence of Salmonella on soft rotted produce compared with that on “healthy” (asymptomatic) samples (9) and promoted the growth of S. enterica beyond levels needed to cause human disease (this work). Thus, soft rot signals an increased food safety risk to consumers. The reduction in soft rot symptoms in the presence of S. enterica is of concern to human health because S. enterica obscures the effects of mishandling or spoilage. Produce perceived to be healthy or with little rot may still contain infectious doses of Salmonella, and its consumption may lead to human disease. Understanding the interactions between bacterial plant and human pathogens on plants and the factors affecting proliferation and persistence will aid strategies for maintaining food safety.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
The bacterial strains used in this study are listed in Table 1. S. enterica sv. Saintpaul XbaI pattern JN6X01.0048 caused the largest salmonellosis outbreak in the United States, involving jalapeño and Serrano peppers and possibly tomatoes (39). Other recent outbreaks of salmonellosis caused by S. enterica sv. Saintpaul followed consumption of contaminated alfalfa sprouts (2003, 2008, and 2009) and cantaloupe (2006) (40). Strains were cultured on Luria-Bertani (LB) agar medium at 37°C. When necessary, antibiotics were added at the following concentrations: chloramphenicol (Chl), 50 µg ml−1; kanamycin (Kan), 50 µg ml−1; nalidixic acid (Nal), 30 µg ml−1. Salmonella-Shigella (SS) agar, a differential selective medium for Salmonella, was used to determine S. enterica populations. E. coli O157:H7 populations were determined on sorbitol-MacConkey (SMAC) agar, a differential selective medium for pathogenic E. coli O157:H7.
TABLE 1.
List of bacterial strainsa
| Strain/serovar | Relevant characteristics | Reference(s) |
|---|---|---|
| E. coli | ||
| O157:H7 strain 96A 13466 Nalr |
Nalr, spontaneous nalidixic acid derivative of outbreak strain clinically isolated from a patient who had consumed unpasteurized apple cider, 1996 |
43, this work |
| P. carotovorum subsp. carotovorum | ||
| WPP359 (WT) | Chlr, gfp::chl-labeled derivative of wild-type strain WPP14, isolated from an infected potato stem in Wisconsin, 2001 |
18 |
| ΔbudB | Kanr, ΔbudB::kan derivative of wild-type strain WPP14 | 22 |
| S. enterica | ||
| Saintpaul | Outbreak strain isolated from contaminated peppers from Mexico, 2008 | 39 |
Nalr, Chlr, and Kanr indicate resistance to nalidixic acid, chloramphenicol, and kanamycin, respectively.
Leaf inoculation.
Heads of romaine lettuce were purchased from a local grocery store in Madison, WI, and used within 48 h of purchase. Overnight bacterial cultures plated on LB were suspended in sterile deionized water and adjusted to an optical density at 600 nm (OD600) of 0.200 ± 0.005. The ten outermost leaves of each head of lettuce were detached and stab-inoculated at the midrib with 1 µl of a 10−2 dilution of each bacterial suspension (~103 CFU) using a 10-µl pipette tip. The pipette tip was positioned perpendicular to the leaf surface, and a 1- to 3-mm puncture was made at the midrib halfway along the length of the leaf. Leaves were inoculated with S. enterica sv. Saintpaul, E. coli O157:H7, WT P. carotovorum subsp. carotovorum, or a P. carotovorum subsp. carotovorum budB mutant. Alternatively, leaves were coinoculated with 103 CFU each of S. enterica or E. coli O157:H7 and WT P. carotovorum subsp. carotovorum or the P. carotovorum subsp. carotovorum budB mutant. Control leaves were mock-inoculated with sterile water. Leaves were incubated at 24°C in GasPak 100 system jars containing GasPak EZ anaerobe container system sachets. According to the manufacturer, anaerobic conditions (≤1% oxygen) are achieved within 2.5 h at 35°C, and within 24 h, the carbon dioxide concentration is ≥13%. These conditions are similar to those reported for lettuce in MAP (41). There were a total of 36 replicates per treatment except in the negative-control group, which contained 12 replicates. The experiment was repeated at least three times.
Alternatively, leaves inoculated with 106 CFU of S. enterica or P. carotovorum subsp. carotovorum or coinoculated with 106 CFU each of S. enterica and P. carotovorum subsp. carotovorum were incubated aerobically in partially unsealed plastic gallon-size zip-top bags at 24°C. A higher inoculum of P. carotovorum subsp. carotovorum was necessary to achieve soft rot disease on 100% of samples because P. carotovorum subsp. carotovorum is less virulent in aerobic conditions (18). Additionally, the plant host is more resistant to phytopathogen infection in aerobic conditions because anaerobiosis impairs plant defenses, such as cell wall lignification, callose deposition, and oxygen-dependent production of phytoalexins, phenolics, and free radicals (18). There were a total of 18 replicates per treatment except in the negative-control group, which contained 6 replicates. The experiment was repeated three times.
Measurement of lesion development and bacterial populations on leaves.
Nine bacterium-inoculated and three control leaves were removed and sampled every 24 h up to 96 h. The length of soft rot lesions (i.e., nonintact tissue) was measured using a standard metric ruler. All lesion lengths smaller than 3 mm were regarded as equal because punctures made in the midrib during inoculation ranged from 1 to 3 mm. For lesions of <3 mm, we were unable to macroscopically differentiate mechanical injury during inoculation and soft rot. Leaf tissue surrounding the point of inoculation (r = 5 mm) was removed using a flame-sterilized core borer and manually macerated in a sterile 1.7-ml microcentrifuge tube. Sterile deionized water equivalent to four times (wt/vol) the tissue sample was added to each tube. This suspension was serially diluted and plated onto selective or differential medium for isolation of the inoculated strains. SS and LB+Kan agar plates were incubated overnight at 33°C. SMAC+Nal plates were kept at 42°C overnight. LB+Chl agar plates were maintained at 37°C for 28 h.
Spectrophotometric determination of pH.
The pH of each leaf sample was determined spectrophotometrically using a NanoDrop ND-1000 spectrophotometer, based on the method of Braun (42). The absorption maxima of the pH indicator dye bromocresol purple (BCP) (0.1% wt/vol) were determined to be 430 nm (yellow) and 588 nm (purple). BCP was selected because preliminary data (not shown) indicated that the pH values in the color change interval for BCP (pH 5.2 to 6.8) are similar to the range of pH values expected in the experiment. Four microliters of the described leaf suspension (see above) was combined with 1 µl BCP, and the absorption of this solution was read at 430 nm and 588 nm. The pHs of eight buffered solutions (pH 4.8 to 9.0) were determined both spectrophotometrically and using an Accumet Research AR15 pH/mV/oC meter. These values were fitted using a linear model described by the equation y = 0.96584x − 0.24483, where x is the value given by the pH meter and y is the spectrophotometrically calculated pH. The pH values reported in this study reflect the spectrophotometrically determined pH values, adjusted for error using this equation.
Proteolytic activity assay.
Lettuce lysate was prepared by crushing the leaves of entire heads of romaine lettuce, collecting the homogenate in 50-ml conical tubes, centrifuging twice at 4,000 × g for 30 min with discard of plant debris in between, and sequentially filtering the supernatant through 0.8-µm and 0.2-µm filters. Fresh leaf lysate was prepared each day.
Bacterial suspensions (OD600 = 0.5) of S. enterica and P. carotovorum subsp. carotovorum were prepared from overnight streak cultures and serially diluted 10−4. Lettuce lysate (1 ml) was inoculated with 100 µl of the diluted suspensions or coinoculated with 100 µl of each of the diluted suspensions (1:1 or 1:10 S. enterica/P. carotovorum subsp. carotovorum ratio), and cultures were incubated either aerobically or anaerobically in GasPak jars for 24 h at 28°C with shaking at 200 rpm. Inoculation with sterile water served as the negative control. There were three replicates per treatment.
Bacterial proteolytic activity was assessed on 3% skim milk agar plates containing 10% lettuce lysate. Lettuce lysate was added to cooled autoclaved milk agar, and 25 ml of medium was dispensed into each petri plate. Plates were prepared on the day of inoculation. Ten microliters of each of the lettuce lysate cultures was spotted onto milk agar plates and dried in the hood. Additionally, lettuce lysate cultures were centrifuged at 13,000 × g for 2 min. The supernatant was filtered through a 0.2-µm filter, and 150 µl of the filtrate was applied to wells in milk agar-lettuce lysate plates. Wells were formed by removal of agar plugs using a flame-sterilized core borer (r = 6 mm). Plates were incubated either aerobically or anaerobically in GasPak jars, identical to their respective lettuce lysate cultures, at 28°C for 48 h, after which the zones of clearing were measured. Bacterial cultures were also serially diluted and plated onto SS agar or LB+Chl and incubated at 37°C for at least 24 h to enumerate bacterial populations. The experiment was repeated three times.
Statistical analyses.
Statistical analyses were performed using R software (version 2.11.1; R Development Core Team, R Foundation for Statistical Computing, Vienna, Austria; http://www.R-project.org). Data from all replicated experiments for a particular parameter (i.e., population, disease progression, pH) were pooled, and differences between the means of the various treatments were analyzed using Tukey’s HSD test (based on a two-way ANOVA), with significance set at a P value of <0.05. Separate Tukey’s HSD tests were conducted for S. enterica, E. coli 157: H7, and P. carotovorum subsp. carotovorum populations in MAP. Single Tukey’s HSD tests were completed at each time point for disease progression and pH in MAP. Data collected at 24 hpi and 48 hpi in aerobic conditions were combined, and each parameter (population, disease progression, pH) was analyzed using a single Tukey’s HSD test. Interactions between treatment and experiment were analyzed using a two-way ANOVA.
SUPPLEMENTAL MATERIAL
S. enterica had no effect on the WT P. carotovorum subsp. carotovorum population, disease progression, or pH in aerobic conditions (A to C). S. enterica achieved higher populations in the presence of WT P. carotovorum subsp. carotovorum (A) but did not acidify the lettuce environment (C). Data shown are the means of nine samples per treatment from a representative experiment. Vertical bars indicate the standard errors. In each panel, 24-hpi and 48-hpi data collected in all three experiments were pooled and analyzed for statistically significant differences using Tukey’s HSD test (n = 212 [A] or n = 216 [B and C]). Download
E. coli O157:H7 had no effect on P. carotovorum subsp. carotovorum growth, disease progression, or pH in microaerophilic conditions (A to C). Data shown are the means of nine samples per treatment from a representative experiment at 72 hpi. Vertical bars indicate the standard errors. In each panel, statistical analyses were performed with data collected from all three experiments using Tukey’s HSD test (n = 53 [A] or n = 90 [B and C]). Download
Growth of S. enterica and P. carotovorum subsp. carotovorum in lettuce leaf lysate.
ACKNOWLEDGMENTS
We thank Linda Sullivan for use of the GasPak 100 system jars and Lisa Gorski, Diana Downs, and Kimberly Cowles for critical review of the manuscript.
This work was supported by USDA-NIFA grant no. 2011-67017-30166.
Footnotes
Citation Kwan G, Charkowski AO, Barak JD. 2013. Salmonella enterica suppresses Pectobacterium carotovorum subsp. carotovorum population and soft rot progression by acidifying the microaerophilic environment. mBio 4(1):e00557-12. doi:10.1128/mBio.00557-12.
REFERENCES
- 1. Barak JD, Schroeder BK. 2012. Interrelationships of food safety and plant pathology: the life cycle of human pathogens on plants. Annu. Rev. Phytopathol. 50:241–266 [DOI] [PubMed] [Google Scholar]
- 2. Batz MB, Hoffmann S, Morris JG. 2011. Ranking the risks: top 10 pathogen-food combinations with the greatest burden on public health. Emerging Pathogens Institute, Gainesville, FL: https://folio.iupui.edu/bitstream/handle/10244/1022/72267report.pdf [Google Scholar]
- 3. Teplitski M, Noel JT, Alagely A, Danyluk MD. 2012. Functional genomics studies shed light on the nutrition and gene expression of non-typhoidal Salmonella and enterovirulent E. coli in produce. Food Res. Int. 45:576–586 [Google Scholar]
- 4. Aruscavage D, Miller SA, Ivey ML, Lee K, LeJeune JT. 2008. Survival and dissemination of Escherichia coli O157:H7 on physically and biologically damaged lettuce plants. J. Food Prot. 71:2384–2388 [DOI] [PubMed] [Google Scholar]
- 5. Yamazaki A, Li J, Hutchins WC, Wang L, Ma J, Ibekwe AM, Yang CH. 2011. Commensal effect of pectate lyases secreted from Dickeya dadantii on proliferation of Escherichia coli O157:H7 EDL933 on lettuce leaves. Appl. Environ. Microbiol. 77:156–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Deering A, Mauer L, Pruitt R. 2012. Internalization of E. coli O157:H7 and Salmonella spp. in plants: a review. Food Res. Int. 45:567–575 [Google Scholar]
- 7. Lindow SE, Brandl MT. 2003. Microbiology of the phyllosphere. Appl. Environ. Microbiol. 69:1875–1883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Barak JD, Liang AS. 2008. Role of soil, crop debris, and a plant pathogen in Salmonella enterica contamination of tomato plants. PLoS One 3:e1657 http://dx.doi.org/10.1371/journal.pone.0001657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wells JM, Butterfield JE. 1997. Salmonella contamination associated with bacterial soft rot of fresh fruits and vegetables in the marketplace. Plant Dis. 81:867–872 [DOI] [PubMed] [Google Scholar]
- 10. Brandl MT. 2008. Plant lesions promote the rapid multiplication of Escherichia coli O157:H7 on postharvest lettuce. Appl. Environ. Microbiol. 74:5285–5289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Noel JT, Joy J, Smith JN, Fatica M, Schneider KR, Ahmer BMM, Teplitski M. 2010. Salmonella SdiA recognizes N-acyl homoserine lactone signals from Pectobacterium carotovorum in vitro, but not in a bacterial soft rot. Mol. Plant Microbe Interact. 23:273–282 [DOI] [PubMed] [Google Scholar]
- 12. Lund BM. 1983. Bacterial spoilage, p 219–257 In Dennis C, Post-harvest pathology of fruits and vegetables. Academic Press, New York, NY. [Google Scholar]
- 13. Forscythe SJ, Hayes PR. 1998. Food hygiene, microbiology, and HACCP, 3rd ed, p 121 Springer Verlag, Gaithersburg, MD [Google Scholar]
- 14. Liao CH, McEvoy JL, Smith JL. 2003. Control of bacterial soft rot and foodborne human pathogens on fresh fruits and vegetables, p 165–193 In Huang HC, Acharya SN, Advances in plant disease management. Research Signpost, Kerala, India [Google Scholar]
- 15. Pérombelon MCM. 2002. Potato diseases caused by soft rot erwinias: an overview of pathogenesis. Plant Pathol. 51:1–12 [Google Scholar]
- 16. Toth IK, Bell KS, Holeva MC, Birch PR. 2003. Soft rot erwiniae: from genes to genomes. Mol. Plant Pathol. 4:17–30 [DOI] [PubMed] [Google Scholar]
- 17. Barras F, van Gijsegem F, Chatterjee AK. 1994. Extracellular enzymes and pathogenesis of soft-rot Erwinia. Annu. Rev. Phytopathol. 32:201–234 [Google Scholar]
- 18. Kim H-S, Thammarat P, Lommel SA, Hogan CS, Charkowski AO. 2011. Pectobacterium carotovorum elicits plant cell death with DspE/F but the P. carotovorum DspE does not suppress callose or induce expression of plant genes early in plant-microbe interactions. Mol. Plant Microbe Interact. 24:773–786 [DOI] [PubMed] [Google Scholar]
- 19. Kariola T, Palomäki TA, Brader G, Palva ET. 2003. Erwinia carotovora subsp. carotovora and Erwinia-derived elicitors HrpN and PehA trigger distinct but interacting defense responses and cell death in Arabidopsis. Mol. Plant Microbe Interact. 16:179–186 [DOI] [PubMed] [Google Scholar]
- 20. Mattinen L, Tshuikina M, Mäe A, Pirhonen M. 2004. Identification and characterization of Nip, necrosis-inducing virulence protein of Erwinia carotovora subsp. carotovora. Mol. Plant Microbe Interact. 17:1366–1375 [DOI] [PubMed] [Google Scholar]
- 21. Pemberton CL, Whitehead NA, Sebaihia M, Bell KS, Hyman LJ, Harris SJ, Matlin AJ, Robson ND, Birch PR, Carr JP, Toth IK, Salmond GP. 2005. Novel quorum-sensing-controlled genes in Erwinia carotovora subsp. carotovora: identification of a fungal elicitor homologue in a soft-rotting bacterium. Mol. Plant Microbe Interact. 18:343–353 [DOI] [PubMed] [Google Scholar]
- 22. Marquez-Villavicencio MdelP, Weber B, Witherell RA, Willis DK, Charkowski AO. 2011. The 3-hydroxy-2-butanone pathway is required for Pectobacterium carotovorum pathogenesis. PLoS One 6:e22974 http://dx.doi.org/10.1371/journal.pone.0022974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kotoujansky A. 1987. Molecular genetics of pathogenesis by soft-rot Erwinias. Annu. Rev. Phytopathol. 25:405–430 [Google Scholar]
- 24. Rojas-Graü MA, Oms-Oliu G, Soliva-Fortuny R, Martín-Belloso O. 2009. The use of packaging techniques to maintain freshness in fresh-cut fruits and vegetables: a review. Int. J. Food Sci. Tech. 44:875–889 [Google Scholar]
- 25. White JN, Starr MP. 1971. Glucose fermentation endproducts of Erwinia spp. and other enterobacteria. J. Appl. Microbiol. 34:459–475 [DOI] [PubMed] [Google Scholar]
- 26. Kyle JL, Parker CT, Goudeau D, Brandl MT. 2010. Transcriptome analysis of E. coli O157:H7 exposed to lysates of lettuce leaves. Appl. Environ. Microbiol. 76:1375–1387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Aruscavage D, Phelan PL, Lee K, LeJeune JT. 2010. Impact of changes in sugar exudates created by biological damage to tomato plants on the persistence of Escherichia coli O157:H7. J. Food Sci. 75:M187–M192 [DOI] [PubMed] [Google Scholar]
- 28. Baker CJ, Atkinson MM, Roy MA, Collmer A. 1986. Inhibition of the hypersensitive response in tobacco by pectate lyase. Physiol. Mol. Plant Pathol. 29:217–225 [Google Scholar]
- 29. Effantin G, Rivasseau C, Gromova M, Bligny R, Hugouvieux-Cotte-Pattat N. 2011. Massive production of butanediol during plant infection by phytopathogenic bacteria of the genera Dickeya and Pectobacterium. Mol. Microbiol. 82:988–997 [DOI] [PubMed] [Google Scholar]
- 30. Ni L, Guo L, Custers JBM, Zhang L. 2010. Characterization of calla lily soft rot caused by Pectobacterium carotovorum subsp. carotovorum bacterial growth and pectate lyase activity under different conditions. J. Plant Pathol. 92:421–428 [Google Scholar]
- 31. Stewart V. 1982. Requirement of Fnr and NarL functions for nitrate reductase expression in Escherichia coli K-12. J. Bacteriol. 151:1320–1325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jones HM, Gunsalus RP. 1987. Regulation of Escherichia coli fumarate reductase (frdABCD) operon expression by respiratory electron acceptors and the fnr gene product. J. Bacteriol. 169:3340–3349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kröger A, Geisler V, Lemma E, Theis F, Lenger R. 1992. Bacterial fumarate respiration. Arch. Microbiol. 158:311–314 [Google Scholar]
- 34. Maynard DN, Barker AV, Minotti PL, Peck NH. 1976. Nitrate accumulation in vegetables. Adv. Agron. 28:71–118 [Google Scholar]
- 35. Cameron AC, Talasila PC, Joles DW. 1995. Predicting film permeability needs for modified atmosphere packaging of lightly processed fruits and vegetables. J. Hort. Sci. 30:25–34 [Google Scholar]
- 36. Gorny JR. 2001. A summary of the CA and MA requirements and recommendations for fresh-cut (minimally processed) fruits and vegetables. Acta Hortic. 600:609–614 [Google Scholar]
- 37. Harris LJ, Farber JN, Beuchat LR, Parish ME, Suslow TV, Garrett EH, Busta FF. 2003. Outbreaks associated with fresh produce: incidence, growth, and survival of pathogens in fresh and fresh-cut produce. Compr. Rev. Food Sci. Food Saf. 2(Suppl):78–141 [Google Scholar]
- 38. United States Department of Agriculture. Economic Research Service 2006. Vegetables and melons outlook, VGS-315. USDA, Washington, DC: http://www.ers.usda.gov/publications/vgs/2006/06jun/vgs315.pdf [Google Scholar]
- 39. Centers for Disease Control and Prevention 2008. Outbreak of Salmonella serotype Saintpaul infections associated with multiple raw produce items—United States. MMWR Morb. Mortal. Wkly. Rep. 57:929-934 [PubMed] [Google Scholar]
- 40. Centers for Disease Control and Prevention. CDC 2012. Multistate foodborne outbreaks. http://www.cdc.gov/outbreaknet/outbreaks.html Accessed 25 January 2012
- 41. Beaudry RM. 1999. Effect of O2 and CO2 partial pressure on selected phenomena affecting fruit and vegetable quality. Postharvest Biol. Technol. 15:293–303 [Google Scholar]
- 42. Braun RD. 1982. An introduction to chemical analysis, p 197–199 McGraw-Hill, New York, NY. [Google Scholar]
- 43. Uljas HE, Ingham SC. 1999. Combinations of intervention treatments resulting in 5-log10 unit reductions in numbers of Escherichia coli O157:H7 and Salmonella typhimurium DT104 organisms in apple cider. Appl. Environ. Microbiol. 65:1924–1929 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
S. enterica had no effect on the WT P. carotovorum subsp. carotovorum population, disease progression, or pH in aerobic conditions (A to C). S. enterica achieved higher populations in the presence of WT P. carotovorum subsp. carotovorum (A) but did not acidify the lettuce environment (C). Data shown are the means of nine samples per treatment from a representative experiment. Vertical bars indicate the standard errors. In each panel, 24-hpi and 48-hpi data collected in all three experiments were pooled and analyzed for statistically significant differences using Tukey’s HSD test (n = 212 [A] or n = 216 [B and C]). Download
E. coli O157:H7 had no effect on P. carotovorum subsp. carotovorum growth, disease progression, or pH in microaerophilic conditions (A to C). Data shown are the means of nine samples per treatment from a representative experiment at 72 hpi. Vertical bars indicate the standard errors. In each panel, statistical analyses were performed with data collected from all three experiments using Tukey’s HSD test (n = 53 [A] or n = 90 [B and C]). Download
Growth of S. enterica and P. carotovorum subsp. carotovorum in lettuce leaf lysate.


