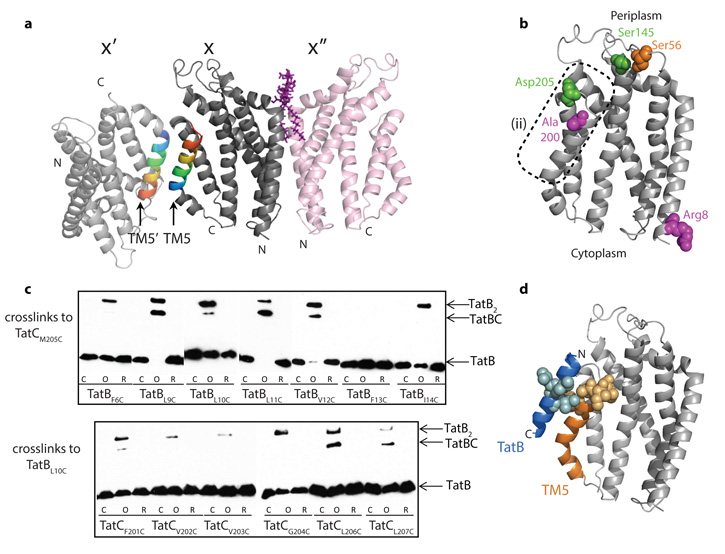
Figure 4. Sites of interaction with other Tat components.
a, AaTatC crystal contacts. The contact between molecules X and X′ involves a packing interaction between antiparallel TM5 helices, whereas that between molecules X and X′′ involves the semi-ordered LMNG molecule shown in purple. b, Positions in EcTatC that have been reported32,34,36 to interact with both TatA and TatB (green), with TatB (magenta), or with TatC (orange) mapped on to AaTatC (full details in Supplementary Table 5). The dotted region corresponds to region (2) in Fig. 2c, d. c, Disulphide crosslinking between E. coli TatB and TatC variants detected by immunoblotting with TatB antibodies. Lanes are untreated (C), oxidized with Cu(II)phenanthroline (O), or oxidized and then reduced with DTT (R). d, The complex between TatC and the transmembrane helix of TatB modelled on the AaTatC X–X′ crystal contact. Positions in EcTatC that form a disulphide bond to a EcTatB L10C variant (yellow), or positions in EcTatB that form a disulphide bond to a EcTatC M205C variant (blue), (data from c) are mapped on to the model.