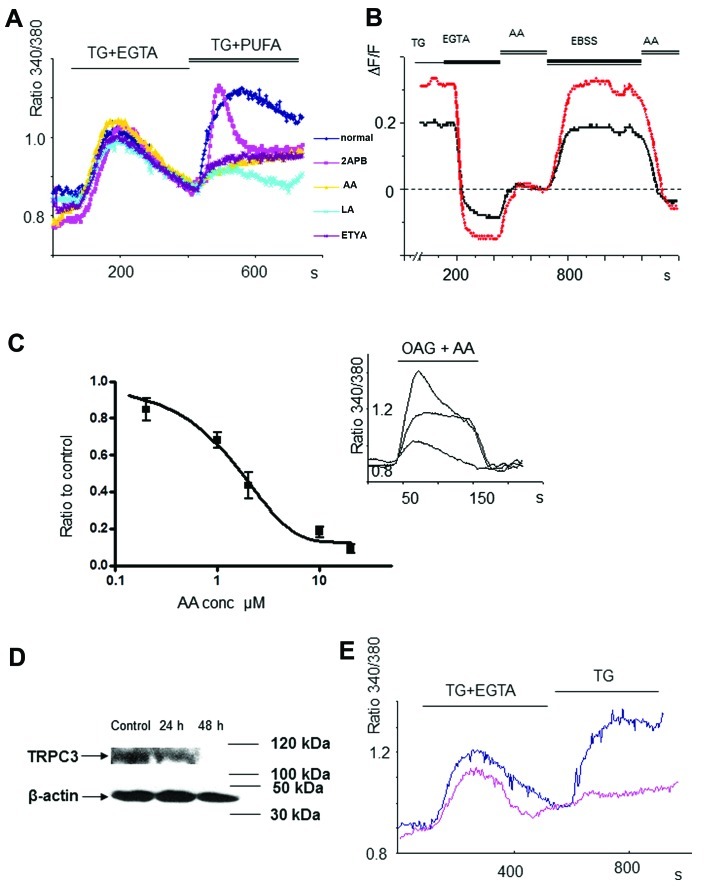
Figure 2.
Polyunsaturated fatty acids inhibited Ca2+ entry through TRPC3 channels. (A) Ca2+ entry was evoked by store depletion with thapsigargin (TG, 1 μM) and EGTA (blue line). 2-APB (pink line, 100 μM) induced a transient Ca2+ elevation following a rapid decrease. [Ca2+]i elevation was inhibited by arachidonic acid (AA, yellow line, 10 μM), linonic acid (LA, 10 μM) and ETYA (brown line, 20 μM). (B) The dashed horizontal line indicates the basal Ca2+ level. TG (1 μM) elevated cellular Ca2+ levels that were rapidly reduced to below basal levels by wash out with EGTA (200 μM). Application of arachidonic acid (AA, 10 μM) prevented the overshooting response seen in (A) and only returned Ca2+ to the basal level, indicating a block of the TRPC-mediated Ca2+ entry pathway by AA. Wash off of AA with EBSS caused an overshooting response to occur that could be blocked, as previously, by application of AA (10 μM). (C) Log dose response curve of varying concentrations of AA upon the OAG-induced Ca2+ elevation. Responses were quantified as the area under the Ca2+ response curve and normalized by determining the area in the presence of AA, expressed as a fraction of the control response. Curve fit was with a single sigmoidal function. The inlet shows an example of different concentration-dependent effects of AA upon Ca2+ entry elicited by OAG (50 μM). (D) Western blot analysis demonstrates that protein levels of TRPC3 channels gradually diminished after 48 h. (E) Ca2+ entry evoked by store depletion was significantly reduced 48 h after incubation of MCF-7 cells with RNAi against TRPC3, . Results were averaged from 3 coverslips except where indicated.