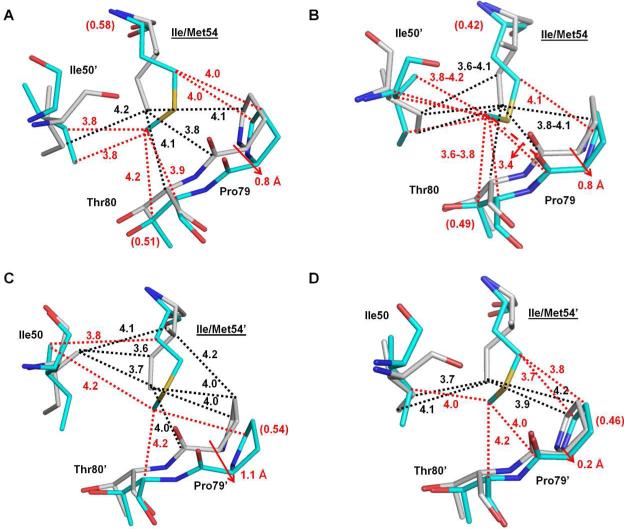
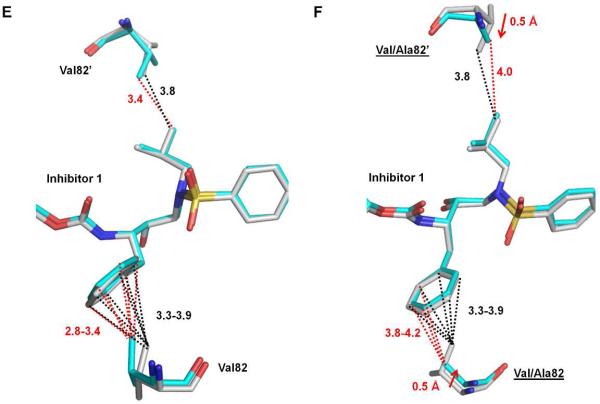
Figure 5.
Structural changes of PRI54M and PRV82A. Carbon atoms are colored gray for PRWT–inhibitor 1 and cyan for PRI54M–inhibitor 1 or PRV82A–inhibitor 1. Interactions are indicated by black lines for PRWT–inhibitor 1 and red lines for the mutants. Van der Waals and C-H...π interactions are indicated by dotted lines. (A-B) Interactions of the major (A) and minor (B) conformations of Ile/Met54 with Ile50’ and 80s loop residues in subunit 1-99. (C-D) Interactions of Ile/Met54’ with Ile50 and the major (C) and minor (D) conformation of 80's loop residues in subunit 1’-99’. Relative occupancies for alternative conformations of residues are shown in parentheses. Red arrows indicate the shifts of the mutants relative to PRWT. (E) Interactions of Val82/82’ with inhibitor 1 in PRWT and PRI54M. (F) Interactions of Val/Ala82/82’ with inhibitor 1 in PRWT and PRV82A.