Table 1.
Substrate generality in the one-pot production the pyrazoles
| entry | R1/R2 (1) | Ar in 5 | 8 | yield 8(%)b |
|---|---|---|---|---|
| 1 | H/Me (1a) | 4-MeOC6H4 (5a) | 8a | 87 |
| 2 | H/Me (1a) | 4-MeC6H4 (5b) | 8b | 91 |
| 3 | H/Me (1a) | 4-ClC6H4 (5c) | 8c | 90 |
| 4 | H/Me (1a) | Ph (5d) | 8d | 89 |
| 5 | H/Me (1a) | 4-NO2C6H4 (5e) | 8e | 71 |
| 6 | H/Me (1a) | 2,4-2ClC6H3 (5f) | 8f | 72 |
| 7 | H/t-Bu (1b) | 4-MeOC6H4 (5a) | 8g | 89 |
| 8 | H/Bn (1c) | 4-MeOC6H4 (5a) | 8h | 88 |
| 9 | Me/Me (1d) | 4-MeOC6H4 (5a) | 8i | 74 |
| 10 | Me/Me (1d) | 4-ClC6H4 (5c) | 8j | 66 |
| 11 | Me/Bn (1e) | 4-MeOC6H4 (5a) | 8k | 69 |
| 12 | Et/Bn (1f) | 4-MeOC6H4 (5a) | 81 | 48 |
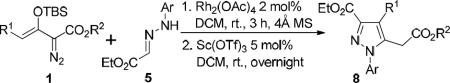
Reactions were carried out on a 0.5 mmol scale: 1 (0.6 mmol), 5 (0.5 mmol), 4 Å MS (100 mg), in 3.0 mL DCM with Rh2(OAc)4 (2.0 mol%) at room temperature; then Sc(OTf)3 (5.0 mol%) was added and stirred at room temperature overnight.
Isolated yield of 8 based on limiting reagent 5.
